The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Basic Research for Clinicians
Designing a Cardiology Research Project – Research Questions, Study Designs and Practical Considerations
Volume 2, Jan 2013
Ann Dewey PhD, Amy Drahota PhD, Carole Fogg MSc, Paul R Kalra MA, FRCP, MD, Sally Kilburn PhD, Christian Markham PhD, Donah Zachariah MBBS, MRCP, Rebecca Stores PhD, Hampshire, UK
J Clin Prev Cardiol. 2013;2(1):37-47
Formulating a Research Question
Millions saw the apple fall but it was Newton who asked why. The greatest discoveries and innovations of all time have stemmed from asking questions. For a researcher, formulating a clear and concise research question is the key to forming a blueprint on which to build the rest of the research process.
The research idea is fundamental. It is crucial that this is of genuine interest to the researcher and is usually a result of past experiences, an awareness of existing knowledge gaps or a desire to discover new solutions to existing problems. Depending on one’s level of expertise in the chosen field, an extensive amount of groundwork and searching of the literature would be required to understand what is already known and what is not. Research should be aimed at generating new information that would otherwise not be available. The proposed research must meet important professional and societal goals, fit with the mission of the organization, garner administrative support, and be accomplished with available resources in a reasonable time frame.
The researcher then aims to construct a research question that is focused and concise. The research question serves two purposes – it determines where and what kind of research is intended, and it identifies the specific objectives the study will address. Research questions are of different kinds (Table 1) but irrespective of the type of question, it should challenge researchers to see matters from a new perspective. They should address an issue, problem or controversy with a conclusion based on the analysis and interpretation of evidence. Properly formulated questions would therefore yield findings that contribute to building a knowledge base that ultimately will inform clinical practice, enhance decisions that improve efficient use of resources and allow application to the wider society.
In constructing a sound research question, the PICO format which was mentioned in the first article of this series (1) is a good framework to use (2). P stands for population or the problem in question, I stands for Intervention (exposure to be considered), C stands for control or comparison treatment/placebo and O for outcome of interest. For example, if the research idea were omega-3 and its effect on arrhythmias post myocardial infarction, the population of interest would be patients’ post myocardial infarction, the Intervention would be administration of omega-3 supplements with the control being those that do not receive omega-3 supplements. The outcome in this scenario would be the impact on arrhythmic burden. A well-formulated research question would therefore incorporate all these factors and would lead to the generation of a hypothesis specifying the nature of the relationships to be observed and measured. (A hypothesis is a declarative sentence that predicts the results of a research study based on existing scientific knowledge and stated assumptions and if true, would explain the researchers’ observations.)
.jpg)
Investigation of the validity of a diagnostic or screening test might merit an alternative format to PICO. In this type of research the examination is seeing if a novel test or assessment accurately identifies people who have the condition of interest from those that do not, culminating in the presentation of sensitivity, specificity, positive predictive and negative predictive values for the test rather than lives saved by the test. An example of this type of research “Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging” by Feuchtner et al. (29) is used to illustrate the essential components below:
It is important to ensure that the research question captivates not just the interest of the researcher but also the reviewer. Once a question has been formulated and a hypothesis generated, it is often sensible to ascertain the feasibility of conducting the project early on to avoid waste of valuable resources in the form of a pilot study.
The services of a biostatistician or a methodologist to guide the researcher in calculating sample sizes and choosing an appropriate study design can be invaluable. This will be discussed in greater detail in due course.
The researcher then aims to construct a research question that is focused and concise. The research question serves two purposes – it determines where and what kind of research is intended, and it identifies the specific objectives the study will address. Research questions are of different kinds (Table 1) but irrespective of the type of question, it should challenge researchers to see matters from a new perspective. They should address an issue, problem or controversy with a conclusion based on the analysis and interpretation of evidence. Properly formulated questions would therefore yield findings that contribute to building a knowledge base that ultimately will inform clinical practice, enhance decisions that improve efficient use of resources and allow application to the wider society.
In constructing a sound research question, the PICO format which was mentioned in the first article of this series (1) is a good framework to use (2). P stands for population or the problem in question, I stands for Intervention (exposure to be considered), C stands for control or comparison treatment/placebo and O for outcome of interest. For example, if the research idea were omega-3 and its effect on arrhythmias post myocardial infarction, the population of interest would be patients’ post myocardial infarction, the Intervention would be administration of omega-3 supplements with the control being those that do not receive omega-3 supplements. The outcome in this scenario would be the impact on arrhythmic burden. A well-formulated research question would therefore incorporate all these factors and would lead to the generation of a hypothesis specifying the nature of the relationships to be observed and measured. (A hypothesis is a declarative sentence that predicts the results of a research study based on existing scientific knowledge and stated assumptions and if true, would explain the researchers’ observations.)
.jpg)
Investigation of the validity of a diagnostic or screening test might merit an alternative format to PICO. In this type of research the examination is seeing if a novel test or assessment accurately identifies people who have the condition of interest from those that do not, culminating in the presentation of sensitivity, specificity, positive predictive and negative predictive values for the test rather than lives saved by the test. An example of this type of research “Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging” by Feuchtner et al. (29) is used to illustrate the essential components below:
- Condition you want to detect (coronary artery disease)
- Population to receive the test (people presenting to emergency departments with chest pain)
- Test of interest or index test (resting myocardial CT perfusion from coronary CT angiography)
- Verification of condition using the gold standard or reference test (SPECT-myocardial perfusion imaging)
- This could be summarized as condition, population, index test, gold standard/reference tests or CPIG.
- The acronym FINER is also a valuable tool to summarize the key characteristics of a good research question (3)
- Feasible (adequate subjects, technical expertise, time and money, and scope)
- Interesting to the investigator
- Novel (confirms or refutes previous findings, provides new findings)
- Ethical
- Relevant (to scientific knowledge, clinical and health policy, future research directions)
It is important to ensure that the research question captivates not just the interest of the researcher but also the reviewer. Once a question has been formulated and a hypothesis generated, it is often sensible to ascertain the feasibility of conducting the project early on to avoid waste of valuable resources in the form of a pilot study.
The services of a biostatistician or a methodologist to guide the researcher in calculating sample sizes and choosing an appropriate study design can be invaluable. This will be discussed in greater detail in due course.
Involving Service Users and Lay Members in the Development of Your Study
A good way of making sure that the research project is applicable and important to the population for whom it is intended to help is to involve patients or carers from the population (“service users”) or members of the public (e.g., charity workers, friends or family) who may have an interest in the condition in the development of the study. You can advertise for volunteers to be involved through specialist clinics or through community organizations or health services, with a clear description of their role and the level of commitment required – for they might be involved in the groups that oversee the study from implementation to dissemination. Such members, also referred to as “Public Patient Involvement” (PPI) members, can give valuable contributions as regards selecting patient-focused outcome measures for the study, reviewing the objectives for appropriateness and patient benefit, helping to develop the intervention (4), and reviewing whether the study methodology will be feasible and acceptable for patients. Therefore, the study quality, ease of implementation and likely impact for patients can be improved by having input and feedback from the very people who are likely to be included in such a study.
Choosing a Study Design
Different research questions require different research designs to answer them. No single design is “better” than another. The design chosen should fit the particular research question. Questions focusing on effectiveness of treatment, cause, prognosis, diagnosis or prevention are usually best answered using deductive approaches to knowledge creation that utilize quantitative designs. In contrast, questions about the experience, attitudes or beliefs around illness are best answered using an inductive approach to knowledge creation utilizing qualitative designs. Many different quantitative and qualitative research designs exist, each with their own purpose and with strengths and limitations.
Empirical research (Fig. 1) is data generated by means of direct and indirect observation or experience which can be analyzed either quantitatively (deduction) or qualitatively (induction). Deduction refers to an approach to research that begins with abstract ideas or general principles and works toward using empirical data to test those ideas. On the other hand induction refers to an approach to research that begins with empirical data and works toward abstract ideas or general principles. Which approach is adopted depends on the type of research question you are seeking to answer. Whether inductive or deductive, there are principles in research design that need to be consistently applied to the processes of sampling, data collection and analysis.
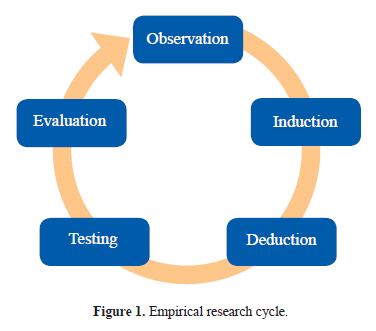
Quantitative research has been defined as “a formal, objective, systematic process in which numerical data are utilized to obtain information about the world” (5). Quantitative research designs may be (a) observational, such as studies describing the incidence or prevalence of diseases or exploring relationships between different factors, or (b) interventional, where different methods of screening, preventing, diagnosing or treating a condition or disease are tested. Descriptions of the most commonly employed designs in cardiology research are provided below.
Empirical research (Fig. 1) is data generated by means of direct and indirect observation or experience which can be analyzed either quantitatively (deduction) or qualitatively (induction). Deduction refers to an approach to research that begins with abstract ideas or general principles and works toward using empirical data to test those ideas. On the other hand induction refers to an approach to research that begins with empirical data and works toward abstract ideas or general principles. Which approach is adopted depends on the type of research question you are seeking to answer. Whether inductive or deductive, there are principles in research design that need to be consistently applied to the processes of sampling, data collection and analysis.

Quantitative research designs
Quantitative research has been defined as “a formal, objective, systematic process in which numerical data are utilized to obtain information about the world” (5). Quantitative research designs may be (a) observational, such as studies describing the incidence or prevalence of diseases or exploring relationships between different factors, or (b) interventional, where different methods of screening, preventing, diagnosing or treating a condition or disease are tested. Descriptions of the most commonly employed designs in cardiology research are provided below.
Observational research designs
Cross-sectional studies
Cross-sectional studies
Cross-sectional studies measure a number of variables of interest in a defined population at one point in time. The data may then be examined to see if there are associations between one variable and another. Cross-sectional studies are typically prevalence studies and often referred to as “taking a snapshot.” They involve identifying a representative (cross-sectional) sample of a population. Recently a cross-sectional study to evaluate the arrhythmias associated with acute severe asthma was conducted among 158 adult patients with asthma and 6303 participants without asthma from the cohort of another ongoing, longitudinal, primary care based study. Tachycardia and premature ventricular contractions (PVC) were found to be more prevalent in patients with asthma (3% and 4%, respectively) than those without asthma (0.6%, p < .001; 2%, p = 0.03, respectively), with an even more pronounced risk in those patients receiving β2-mimetics. The prevalence of QTc interval prolongation was however similar in both groups (6). The advantages of cross-sectional studies are that they are typically quicker and cheaper to carry out than other designs and do not involve loss of participants to follow up. Limitations include the fact that risk factors and outcomes are measured at the same time, and therefore questions relating to causality are not able to be investigated. Undiagnosed or diseases of short duration may be missed and only limited data on rare diseases may be produced.
Cohort studies
If a researcher is interested in the likelihood of an individual developing an outcome in relation to their exposure to a specific factor, they may set up a cohort study. Cohort studies are forward looking (prospective) and involve a group of individuals being followed over time to determine who does/does not develop the outcome of interest in relation to and who is/is not exposed to a particular factor.
For example, in a recent study aimed at determining the relationship between left ventricular scar and ventricular repolarization in patients with coronary artery disease (CAD), 64 patients with implantable cardioverter defibrillator (ICD) who had undergone late gadolinium enhancement cardiac magnetic resonance (CMR) imaging prior to device implantation constituted the cohort. The study aim was to evaluate the relationship between known markers of ventricular repolarization that have an association with sudden cardiac death (corrected QT interval, QT dispersion and Tpeak to T-end interval), and the extent and distribution of left ventricular scar in patients with CAD at high SCD risk. For this, scar was quantified using CMR images and repolarization parameters measured on an electrocardiogram performed prior to ICD implantation. This pilot study showed a strong association between limited subendocardial LV scar and prolonged QTc, QTD, and Tpeak-end but no association between any of these repolarization markers and the delivery of appropriate ICD therapy (7).
Advantages of the cohort design include the fact that it is possible to ascertain the time sequence of events clearly, multiple outcomes can be examined with a single exposure and it is a more acceptable design ethically for certain questions. The disadvantages include the expense and time required to follow up large numbers of participants, rare outcomes require large sample sizes and attrition rates can be high. Care also needs to be taken in interpretation of the results, as confounding by disease severity may lead to apparent associations between exposure and outcome which do not truly have a genuine causal pathophysiological relationship.
For example, in a recent study aimed at determining the relationship between left ventricular scar and ventricular repolarization in patients with coronary artery disease (CAD), 64 patients with implantable cardioverter defibrillator (ICD) who had undergone late gadolinium enhancement cardiac magnetic resonance (CMR) imaging prior to device implantation constituted the cohort. The study aim was to evaluate the relationship between known markers of ventricular repolarization that have an association with sudden cardiac death (corrected QT interval, QT dispersion and Tpeak to T-end interval), and the extent and distribution of left ventricular scar in patients with CAD at high SCD risk. For this, scar was quantified using CMR images and repolarization parameters measured on an electrocardiogram performed prior to ICD implantation. This pilot study showed a strong association between limited subendocardial LV scar and prolonged QTc, QTD, and Tpeak-end but no association between any of these repolarization markers and the delivery of appropriate ICD therapy (7).
Advantages of the cohort design include the fact that it is possible to ascertain the time sequence of events clearly, multiple outcomes can be examined with a single exposure and it is a more acceptable design ethically for certain questions. The disadvantages include the expense and time required to follow up large numbers of participants, rare outcomes require large sample sizes and attrition rates can be high. Care also needs to be taken in interpretation of the results, as confounding by disease severity may lead to apparent associations between exposure and outcome which do not truly have a genuine causal pathophysiological relationship.
Case-control studies
When the outcome of interest is rare or takes a long time to develop, a case-control design is often used. An example is a recent study designed to identify potential links between hemoglobin disorders and atherosclerotic disease in South Asians. Hemoglobin abnormalities were identified by mass spectrometry and risk factors compared between patients (8).
Within a case-control study, individuals with the outcome of interest (cases), in this case those with cardiovascular disease (CVD) and individuals without the outcome of interest (controls) are identified by means of predetermined parameters (this study used carotid artery intima thickness as a marker of vascular damage). The researcher then determines whether they have had previous exposure to a causative agent and are therefore backward looking (retrospective). The cases and controls are matched on important variables that may influence the outcome (e.g., age, sex, additional health conditions) so the groups are as similar as possible and the specific effect of the causative agent on the outcome can be more confidently explored. The strengths of this design include the fact that it allows the assessment of causation when the outcome is rare or takes a long time to develop, all the data are collected at one point in time so it is relatively quick, easy and cheap, and it is possible to investigate many risk factors or exposures at one time. The limitations are the difficulties in establishing that the exposure actually occurred before the outcome, obtaining accurate information about exposure to a causative agent which has occurred in the past (i.e., it relies on people’s accuracy or on completeness and accuracy of medical records) and identifying a control group that is similar in all other factors that may have influenced the outcome.
Within a case-control study, individuals with the outcome of interest (cases), in this case those with cardiovascular disease (CVD) and individuals without the outcome of interest (controls) are identified by means of predetermined parameters (this study used carotid artery intima thickness as a marker of vascular damage). The researcher then determines whether they have had previous exposure to a causative agent and are therefore backward looking (retrospective). The cases and controls are matched on important variables that may influence the outcome (e.g., age, sex, additional health conditions) so the groups are as similar as possible and the specific effect of the causative agent on the outcome can be more confidently explored. The strengths of this design include the fact that it allows the assessment of causation when the outcome is rare or takes a long time to develop, all the data are collected at one point in time so it is relatively quick, easy and cheap, and it is possible to investigate many risk factors or exposures at one time. The limitations are the difficulties in establishing that the exposure actually occurred before the outcome, obtaining accurate information about exposure to a causative agent which has occurred in the past (i.e., it relies on people’s accuracy or on completeness and accuracy of medical records) and identifying a control group that is similar in all other factors that may have influenced the outcome.
Interventional research designs
Randomised controlled trial (RCT)
The randomised controlled trial (RCT) is methodologically the strongest design for answering questions relating to effectiveness of treatment. Typically, in an RCT, participants are randomly allocated to receive a new intervention (experimental group) or to receive a conventional intervention or no intervention at all (control group). Because group allocation is down to chance alone, the only systematic difference between the groups should be the intervention. Researchers follow participants over time and then assess whether they experience a specific outcome.
For example, the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) was designed to evaluate the effect of eplerenone on mortality and morbidity in patients with chronic systolic HF in NYHA class II. Patients were randomized 1:1 to double-blind eplerenone or placebo in addition to standard chronic HF therapy and doses of eplerenone adjusted from 25 mg every other day to 50 mg daily, depending on serum potassium. The primary endpoint was a composite of time to cardiovascular death or first hospital admission for worsening HF, whichever occurred first (9).
An RCT may involve a parallel or crossover design. A parallel RCT involves participants being split into two (or more) groups and each treated differently and measurements of the outcomes taken at baseline and at the end of the intervention. A crossover RCT involves participants being split into two or more groups, baseline measures taken, one group given the intervention and the other the comparison; outcomes are measured; there is then a washout period, the groups swap over so that the first group receives the comparison and the second receives the intervention and outcomes are measured again. The main advantages of a crossover design are that each patient serves as their own control, thus minimizing confounding, and that fewer patients are required in the study for equivalent precision of the results, thus making recruitment periods shorter and producing more timely final study results.
For example, the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) was designed to evaluate the effect of eplerenone on mortality and morbidity in patients with chronic systolic HF in NYHA class II. Patients were randomized 1:1 to double-blind eplerenone or placebo in addition to standard chronic HF therapy and doses of eplerenone adjusted from 25 mg every other day to 50 mg daily, depending on serum potassium. The primary endpoint was a composite of time to cardiovascular death or first hospital admission for worsening HF, whichever occurred first (9).
An RCT may involve a parallel or crossover design. A parallel RCT involves participants being split into two (or more) groups and each treated differently and measurements of the outcomes taken at baseline and at the end of the intervention. A crossover RCT involves participants being split into two or more groups, baseline measures taken, one group given the intervention and the other the comparison; outcomes are measured; there is then a washout period, the groups swap over so that the first group receives the comparison and the second receives the intervention and outcomes are measured again. The main advantages of a crossover design are that each patient serves as their own control, thus minimizing confounding, and that fewer patients are required in the study for equivalent precision of the results, thus making recruitment periods shorter and producing more timely final study results.
The unit of randomization can be an individual or larger in nature, for example, a clinic or hospital (a “cluster” design). The most important aspect of RCTs is the random allocation of participants to group helps ensure the groups are similar in all respects except exposure to the intervention. This, alongside the longitudinal nature of the study where by exposure to the intervention precedes the development of the outcome, ensures that any differences in outcome can be attributed to the intervention. The disadvantages of RCTs include the high cost of conducting a trial, sometimes long period of followup and the possibility that individuals who agree to participate in a trial may differ from those to whom the results would be applied.
Non-randomised controlled trial
In some cases, it may not be ethical or feasible to randomly allocate participants to an experimental or control group. In these cases, a less rigorous design such as a non-RCT may be more appropriate. This study design is similar to the RCT in that there are comparison groups who receive and do not receive an intervention and they are followed up over time to determine who does and does not experience specific outcomes. The important difference between the two designs is the absence of random allocation to study group in the non-RCT. In this example studying the effects of bosentan on severe pulmonary hypertension in high-risk candidates for heart transplantation, the control group was formed by those in the original cohort who declined intervention – an illustration of participants “selecting themselves” (10).
In a non-RCT,, participants may select themselves or are selected by a clinician to receive the intervention or not. This is an important limitation because groups may differ in ways other than exposure to the intervention and group difference in outcome at the end of the study may be due to differences in the groups that existed before the intervention began. The intervention therefore may appear to have had an effect on the outcome when, in fact, it was the initial difference in groups that influenced the outcome.
As stated earlier, choice of research design very much depends on the type of research question being asked.
Qualitative research
Qualitative research sets out to explore how people interpret their experiences and the world around them. Qualitative research is useful to explore research questions where knowledge, attitude and behavior patterns are important. While quantitative research questions might ask “how much” and “how long,” qualitative research will ask different questions such as “what does it feel like” or “what do you think about X.” From these unique descriptions, meaning can be extracted and compared with those of others and theories developed to explain behaviors described. For example, a psychosocial research question to be answered using qualitative research might include “what are the barriers that influence use of health services by people with angina’’ (11). Exploring such issues from the patient perspective can help provide direction when planning and implementing appropriate care.
Unlike quantitative methods that use statistical procedures or other means of quantification, the unit of meaning of qualitative research is that of words, not numbers (12). The data are collected in natural setting (such as people’s homes) rather than in controlled settings like laboratories. There are a number of common data collection methods used to collect qualitative research data which are summarized in Table 2 including a brief overview of the strengths and challenges of each approach.
Whichever approaches to data collection are used, qualitative research also recognizes that there are different perspectives (or multiple realities) and welcomes stories and accounts in the participant’s own words which reflect their own personal experience. It accepts that people see and experience things in different ways and that when they share their view of the world it is likely to be subjective, rather than an objective account which collects detailed information or facts. For example, a patient’s view of their treatment may not be recognized by those delivering care. Patients may describe experiences that appear inaccurate to the health provider. Nevertheless, this is how the patient views the experience and a lot can be learned from hearing their “perspective,” which can help to provide understanding and meaning. Qualitative research can, indeed, help sensitize health workers and others to the patient’s everyday experiences.
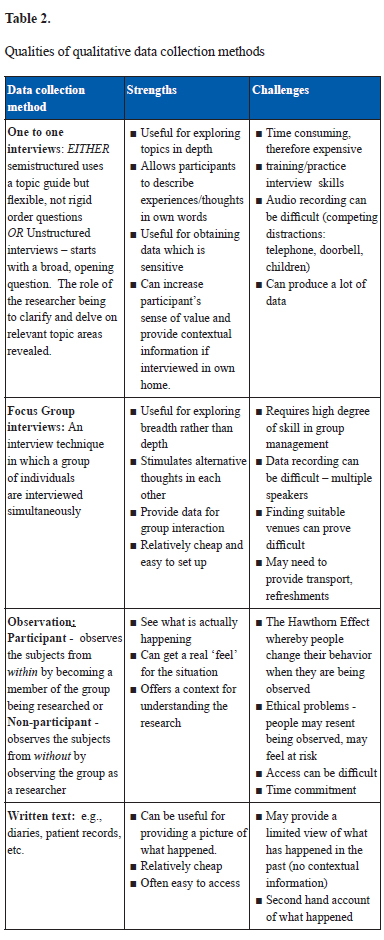
Unlike quantitative methods that use statistical procedures or other means of quantification, the unit of meaning of qualitative research is that of words, not numbers (12). The data are collected in natural setting (such as people’s homes) rather than in controlled settings like laboratories. There are a number of common data collection methods used to collect qualitative research data which are summarized in Table 2 including a brief overview of the strengths and challenges of each approach.
Whichever approaches to data collection are used, qualitative research also recognizes that there are different perspectives (or multiple realities) and welcomes stories and accounts in the participant’s own words which reflect their own personal experience. It accepts that people see and experience things in different ways and that when they share their view of the world it is likely to be subjective, rather than an objective account which collects detailed information or facts. For example, a patient’s view of their treatment may not be recognized by those delivering care. Patients may describe experiences that appear inaccurate to the health provider. Nevertheless, this is how the patient views the experience and a lot can be learned from hearing their “perspective,” which can help to provide understanding and meaning. Qualitative research can, indeed, help sensitize health workers and others to the patient’s everyday experiences.

Sampling
How many interviews or observations or sections of written text will be required? There are no statistical calculations for working out qualitative sampling frameworks and unlike quantitative research you will not usually look for random sampling of the whole population. Instead for a qualitative approach you would normally adopt a purposeful sampling strategy. This means targeting the participants whom you think would be most informative for the purpose of the research question. For example, if you are interested in patients who have recovered from a heart attack, you may want to focus on those recently recovering. “Recently” could be defined as being within the last 6 to 12 weeks; data collection could be restricted to the females in the group. Careful thought thus needs to be given to the characteristics of the target population.
Data Analysis
Data analysis involves making sense of the data collected and starts at the point of collection of the data. Interviews, observations and records that are being maintained will need to be constantly reviewed since this might generate additional questions that need to be asked. If you have produced an audio or video recording, it will be necessary to convert these to a written format. All this can be very time consuming and the process of transcribing and carrying out analysis should never be underestimated (45 minutes tape recording can take up 6–8 hours transcribing, particularly if there are multiple speakers or recording difficulties). External assistance may be useful for this, for example, utilizing the services of transcription companies. However, do bear in mind that typing up the data yourself provides another opportunity to familiarize yourself with what occurred as well as remind yourself who said what, particularly if you have conducted a number of interviews. Even if another person does type up the interviews, it is a good practice to listen again to the audio tapes while reading the typed transcriptions to add in any intonation of voice, correct inaccuracies, etc.
Qualitative data analysis is a creative process with no right way to analyze qualitative data. There are many different approaches available. However, there are different “schools of thought” or theoretical approaches to qualitative analysis. What is important is to be true to the method or philosophical basis adopted (5). Whichever approach is adopted, there are three broad activities to be carried out, namely (a) data reduction (a process of selecting, focusing, simplifying, abstracting and transforming raw data), (b) data display (organizing the data so that conclusions can be drawn using, where appropriate, networks, graphs or charts to clarify main direction of analysis); and finally (c) conclusion drawing/verification (deciding what things mean – regularities, patterns, explanations, possible configurations and verification whereby you ensure your conclusions are plausible, sturdy and confirmed) (13). There are a number of qualitative computer software packages that can help you to organize and speed up the mechanics of analysis, but also allowing you to produce categories, and copies of the data as necessary. Many will produce some impressive diagrams to display your concepts and categories. Unfortunately they do not do the actual analysis for you! If you are interested in hearing more about the value and contribution to the evidence base of qualitative research do read the article “Papers that go beyond numbers (qualitative research)’’ (14). Another useful resource is that of Patton’s Textbook Qualitative Research & Evaluation (15), which provides a comprehensive and systematic review of qualitative methods with many examples and stories illuminating all aspects of qualitative inquiry.
Finally, what are the skills you will require to be an effective qualitative researcher? You will need to be a good listener, non-judgmental, friendly and flexible to able to think abstractly with astute powers of observation and interaction skills. You will need an open mind, be mindful of your preconceived ideas and concepts, so as to interpret the meaning of the data recorded and not make assumptions or draw conclusions on what you expect or want to hear.
As stated earlier, the choice of research design very much depends on the type of research question being asked. Whether quantitative or qualitative, both approaches require a clear question to be asked which uses a sensible methodology to answer the question, ensures a rigorous and systematic data collection and analysis followed by explanation and interpretation of the data. Table 3 summarizes the key differences between the two research paradigms – quantitative and qualitative.
.jpg)
.jpg)
How Do I Find Out Which Research Is Already Planned or In Progress?
In addition to sourcing already published studies to inform the design and objectives of your study, it is also important to check which research is in progress. This will avoid duplication of effort by replicating the study, and perhaps identify multicenter studies that you may want to participate in.
Much progress has been made in encouraging investigators who are planning RCT to register them in the public domain. Trials are allocated a unique International Standard Randomised Controlled Trial Number (ISRCTN) and listed in a register, currently holding more than 11,000 trials (http://www.controlled-trials.com/isrctn/). The register includes details such as the study title, hypothesis, study objectives and eligible population, methodology and main outcomes. Studies from academic, clinical and industry sectors may all be registered. The controlled trials site also hosts other trial registers, such as the National Institutes of Health (NIH), which is also available on http://www.clinicaltrials.gov/. The World Health Organisation (WHO) brings together a network of trial registries from different countries or global regions, and these can be accessed at http://www.who.int/ictrp/network/en/. The International Committee of Medical Journal Editors (ICMJE) is now encouraging investigators to publish their research protocols, for example, in BioMed Central (http://www.biomedcentral.com/authors/protocols). This aids transparency of the research process, as readers are able to compare what was originally intended in the protocol with the final publication, and is also a useful source of information for planned and ongoing research, in addition to methodological considerations.
For study designs other than trials, information is not currently as well coordinated, but there are some avenues to follow. As mentioned in the previous paper (16), planned and ongoing systematic reviews can be registered with PROSPERO (http://www.crd.york.ac.uk/Prospero) and searched using the “search facility” on the website. There is much current discussion on the registration of observational studies (17–19), and such studies are now beginning to be registered at www.ClinicalTrials.gov and on some registers on the WHO register network site. Some journals have begun to publish observational study protocols, for example, the International Journal of Epidemiology. In time, this is likely to become more widespread.
Grant-giving bodies such as charities, specialist clinical societies and governmental funding schemes often list the research they have funded on their websites, for example, the Bill and Melinda Gates Foundation have more than 9000 projects listed (http://www.gatesfoundation.org/grants/Pages/search.aspx). Searches can be refined by topic area, region and year of funding, or more specifically using keywords.
Next Step: How to Design and Write a Research Protocol
As this discussion shows, this stage of the research process is complex, involving numerous people, decisions and processes, because the research question is starting to be turned into a reality. This is why writing a detailed, systematic and clear research protocol is so important. The research protocol, also known as a research proposal, operationalizes the research question into a potentially fundable and successful research project likely to provide an answer to the question. The research protocol outlines the entire project, from the question to intended publication of findings and is required for a range of purposes such as funding and research governance bodies and not least the research team, who will use it to guide the progress and conduct of their study.
If the answer is yes to all three questions, then the protocol is a success and so too may be the next step – the research itself.
Consequently, the research protocol contains numerous sections, some of which are common to all research projects and others that are specific to particular research designs. The key area for variation is within the research methods section, where details will vary according to the research methodology used. There is not space here to explore all these alternatives, but the following link provides a good, generic example of a protocol: http://www.who.int/rpc/research_ethics/guide_rp/en/index.html
Common to all research protocols, though, are the following sections:
- information on the background literature
- a clearly stated research question or hypothesis
- the overall aim of the proposed study and the objectives underpinning it
- a detailed section covering the overall research design and methods for sampling, collecting and analyzing data
- a plan to disseminate the findings from the study as widely as possible
- detailed information on the cost of the study
- the ethical implications and procedures to be adopted by the study
- details of the study team
- a clearly articulated and realistic project management plan
Writing a research protocol is thus an involved task that requires a breadth and depth of expertise and successful studies are often built around a team of people. Although a lead researcher might be the first person to draft a research protocol around an area of interest to them, they will need to involve the expertise of numerous other people in order to conduct a credible and rigorous study. For example, a cardiologist may think of a valuable epidemiological study, but will benefit from the contribution of perhaps medical statisticians and research methodologists in designing and therefore writing the methods section of the protocol. Clinicians may not appreciate the costs involved in delivering their study and can therefore seek the contribution of finance officers in preparing the budget sections of a protocol. Additional valuable and increasingly sought after members of the team are patient and public representatives. A study’s success can be contributed to from the advice of people who experience or provide care and support for people with the conditions and interventions that may be studied. Therefore it is prudent and increasingly an expectation that patient and public involvement (PPI) is an integral part of the development of a protocol and continues through to the dissemination of a study’s findings. PPI, for example, has the potential to contribute valuable insights while designing the recruitment and data collection procedures within a protocol. The Participant Information Sheet (PIS) which should be included in the protocol is likely to be much more understandable and easy to read if it is written in collaboration with service users, which will in turn facilitate the recruitment process for the study and help the investigators adhere to the principles of informed consent. Building a team of researchers with complementary expertise, therefore, not only reduces the burden of preparing a detailed research protocol, but also increases the likelihood of a successful and valuable study, which at the very least may be more likely to receive funding.
The completed draft protocol is now the blueprint for a study designed to answer the question, but it can still be refined further through the process of peer review. Peer review has numerous meanings, but within the arena of research design it is the process of gaining evaluation of an idea and the protocol designed to answer it. Peer review is an opportunity to receive feedback on the quality of the protocol from qualified individuals. A reviewer’s qualifications may arise from their own related work in a similar area of study, to distinct methodological expertise in any area of the protocol. Reviewers provide an objective evaluation of a proposed study and in many cases, additional ideas or information to amend the protocol and therefore improve it. In the case of protocols submitted to funding agencies, peer reviewers also provide an opinion on whether the proposed study should be funded. This, perhaps more than anything, underlines the importance of spending time to build a team, who develop a detailed and crafted research protocol, because unfunded protocols do not answer research questions.
In summary, then, a research protocol provides a pathway to implement a study to answer a defined question. Its development is iterative and requires time, planning and a team with varied expertise, relevant to the research question and design. Peer review is often the final stage in preparing a protocol and Crombie and Florey (20) point out three critical things reviewers often look for in a protocol:
- Is the answer to the question worth having?
- Does the protocol demonstrate a study that is realistic and likely to succeed?
- Does the protocol demonstrate that the study provides value for any investment in it?
If the answer is yes to all three questions, then the protocol is a success and so too may be the next step – the research itself.
Choosing Study Outcomes
A good deal of thought needs to go into choosing the outcomes you will study. Firstly you need to consider who will be the consumers of your research and what do they consider to be the important outcomes. Consumers may include patients, carers, healthcare professionals, policy makers and other researchers; these are the people for whom you want your research to have an impact on. It is good practice to involve patients and public in the specification of outcomes, to ensure that your study will address their needs and wishes. For example, a cardiac patient may not be too concerned as to whether an intervention improves their outcome on a particular medical test; instead they may want to know if it will improve their daily functioning so that they can walk to the shops and back, and improve their quality of life. Consider local support groups, charitable organizations, INVOLVE (http://www.invo.org.uk) and the UK Database of Uncertainties about the Effects of Treatments (DUETs - http://www.library.nhs.uk/duets/SearchResults.aspx?catID=14491) for guidance. INVOLVE offer guidance for how to engage with the public when undertaking research, and you can even post your research project on the INVOLVE research project database as a means of attracting further public involvement. DUETs is a database of questions posed by patients, carers, professionals, research recommendations and ongoing research. For example, there are currently 256 searchable records listed under the category for “cardiovascular diseases,” which can help you determine important outcomes and whole questions which need addressing.
In choosing your outcomes, you should also consider if there are any agreed “core outcome sets” for the condition you are investigating. The COMET (Core Outcome Measures in Effectiveness Trials) Initiative website is a good resource to help with this (http://www.comet-initiative.org). Core outcome sets are an agreed standardized set of outcomes, which should be measured and reported in clinical trials for specific conditions. A quick search under the health area “heart and circulation” on the COMET website generated five useful resources for core outcome sets in this field (21–25). These core outcome sets make it easier for researchers to collate studies in systematic reviews, in order that we can learn more about the body of evidence in a particular field. If your area of study has an identified core outcome set, you should include these outcomes as a minimum, and may consider including other outcomes as relevant.
It is vital that you collect and report on all important outcomes and that you do not fall into the dangerous territory of collecting information on absolutely everything you can think of and only reporting on the things that are significant. The latter approach is problematic for two main reasons: the more tests you run for statistical significance, the more likely you are to find a significant result by chance alone – therefore you should only select the most important outcomes to collect, analyze, and report; and if you only report the significant findings, you are generating bias within the body of the evidence (selective reporting bias or publication bias), which results in a misrepresentation of which interventions are unhelpful (and knowing what does not work is just as important as knowing what does!).
There are tools to measure all sorts of outcomes, from the more objective (e.g., heart rate) to subjective (e.g., quality of life). In selecting your outcomes you need to think carefully about using a reliable and valid tool. So, rather than making up your own questionnaire, first check to see if there is an “off-the-shelf” tool, which has been developed and validated for use in the population you are looking to study. Not only can this save you time, but it will also facilitate the comparison and synthesis of your research with other studies in the field.
Conclusions
Although it is tempting to launch in to data collection for topics of potential research interest, it is vitally important to take time to think clearly about what research you would actually like to do, the most appropriate study design and data to be collected, and consult as widely as you can with potential stakeholders throughout the process of development. Good planning in the beginning and a solid, detailed protocol avoids confusion and error later on in the research process. In the last article of the series, we will consider some of the practical aspects of implementing the finalized research protocol – “making it happen.”
Conflict of Interest
None.
Acknowledgements
The authors would like to thank Dr. Heather Mackenzie for her support and useful comments on the manuscript.
References
References
- Burnell KJ, Charlesworth GM, Sullivan T, Poland F, Orrell M. Involving service users in the development of the SHIELD Carer Supporter Programme. 2012 Health Expectations. doi: 10.1111/hex.12012.
- Cieza A, Ewert T, Ustun TB, Chatterji S, Kostanjsek N, Stucki G. Development of ICF Core Sets for patients with chronic conditions. J Rehabil Med. 2004; 44(Suppl.) 09-Nov.
- Cormack, D. The Research Process in Nursing (4th ed.). Oxford: Blackwell Oxford; 2000.
- Crombie IK, du V Flory C. The Pocket Guide to Grant Applications. London: BMJ Books; 1998.
- Feuchtner GM, Plank F, Pena C, Battle J, Min J, Leipsic J, Labounty T, Janowitz W, Katzen B, Ziffer J, Cury RC. Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging. Heart. 2012;98:1510–7.
- Fogg C, Zachariah D, Markham C, MacKenzie H, Stores R, Kalra P. An introduction to evidence-based practice for cardiologists: finding, utilizing and planning to conduct research to inform practice. J Clin Prev Cardiol. 2012;1:144–51.
- Greenhalgh T , Taylor R. Papers that go beyond numbers (qualitative research). BMJ. 1997;315:740–3.
- Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing Clinical Research (3rd ed.) Lippincott Williams and Wilkins; 2007.
- Leem J, HeeKoh E, Jeong E. Prevalence of angiographically defined obstructive coronary artery disease in asymptomatic patients with type 2 diabetes according to the coronary calcium score. Intern Med. 2012;51:3017–23.
- Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, et al. Standardized endpoint definitions for Transcatheter Aortic Valve Implantation clinical trials: a consensus report from the Valve Academic Research Consortium. i. 2011;57:253–69.
- Loder E, Groves T, MacAuley D. Registration of observational studies. BMJ. 2010;340(c950):375–6.
- Loomis D. Journal Requirements to Register Observational Studies: OEM’s Policy. Occupational and Environmental Medicineoem.bmj.com Occup Environ Med. 2011;68:83–4.
- MacKenzie H, Dewey A, Drahota A, Kilburn S, Kalra P, Fogg C, Zachariah D. Systematic reviews: what they are, why they are important, and how to get involved. J Clin Prev Cardiol. 2012;1:189–98.
- Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei Cas L. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function Circ Heart Fail. 2012;5:54–62.
- Nedeltchev K, Pattynama PM, Biaminoo G, Diehm N, Jaff MR, Hopkins LN, Ramee S, van Sambeek M, Talen A, Vermassen F, Cremonesi A. Define Group. Standardized definitions and clinical endpoints in carotid artery and supra-aortic trunk revascularization trials. Catheterization Cardiovasc Interven. 2010;76:333–44.
- Noyes K, Veazie P, Hall WJ, Zhao H, Buttaccio A, Thevenet-Morrison K, Moss AJ. Cost-Effectiveness of Cardiac Resynchronization Therapy in the MADIT-CRT Trial. J Cardiovasc Electrophysiol. 2012 Aug 22.doi: 10.1111/j.1540-8167.2012.02413.x.
- Patel JV, Chackathayil J, Gammon B, Tracey I, Lovick A, Gill PS, Barejee A, Scargg CA, Scriven JH, Lip GY, Hughes EA. Is the higher risk of cardiovascular disease amongst South Asian populations linked to abnormalities of haemoglobin? A preliminary case control study. Arthersclerosis. 2012 Oct 25. pii: S0021-9150(12)00635-1.
- Patton MQ. Qualitative Research and Evaluation (third edn). USA, CA: Sage; 2001.
- Perez-Villa F, Farrero M, Cardona M, Castel MA, Tatier I, Penela D, Vallejos I. Bosentan in heart transplantation candidates with severe pulmonary hypertension: efficacy, safety and outcome after transplantation. Clin Transplant. 2012 Aug 2. doi: 10.1111/j.1399-0012.2012.01689.x.) [Epub ahead of print]
- Rovellini A, Graziadei G, Folli C, Brambilla AM, Cosentini R, Canetta C, Monzani V. Causes and correlates of anemia in 200 patients with acute cardiogenic pulmonary edema. Eur J Inter Med. 2012;23:733–7.
- Sackett D, Richardson WS, Rosenburg W, Haynes RB. How to Practice and Teach Evidence Based Medicine (second ed.). Churchill Livingstone; 1997.
- Scott PA, Rosengarten JA, Shahed A, Yue AM, Murday DC, Roberts PR, Peebles CR, Harden SP, Curzen NP, Morgan JM. The relationship between left ventricular scar and ventricular repolarization in patients with coronary artery disease: insights from late gadolinium enhancement magnetic resonance imaging. Europace. 2012 Nov 9. [Epub ahead of print]
- Silverman D. Qualitative Research (third edn.). Sage: London; 2011.
- Spragg DD, Dong J, Fetics BJ, Helm R, Marine JE, Cheng A, Henrikson CA, Kass DA, Berger RD. Optimal left ventricular endocardial pacing sites for cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2010;56:774–81.
- Strauss & Corbin. Basics of Qualitative Research and Procedures for Developing Grounded Theory (second edn.).London: Sage; 1998.
- Tod A, Read C, Lacey A. Barriers to uptake of services for coronary heart disease: qualitative study. BMJ. 2001;326(7306).
- Timaran CH, McKinsey JF, Schneider PA, Littooy F. Reporting standards for carotid interventions from the Society for Vascular Surgery. J Vasc Surg. 2011;53:1679–95.
- Warnier MJ, Rutten FH, Kors JA, Lammers JW, de Boer A, Hoes AW, de Bruin ML. Williams. Cardiac arrhythmias in adult patients with asthma. J Asthma. 2012;49:942–6. doi: 10.3109/02770903.2012.724132.
- Weigl M, Cieza A, Andersen C, Kollerits B, Amann E, Stucki G. Identification of relevant ICF categories in patients with chronic health conditions: a Delphi exercise. J Rehabil Med. 2004; 44(Suppl) Dec-21. DOI: 10.1080/16501960410015443
- Williams RJ, Tse T, Harlan WR, Zarin DA. Registration of observational studies: Is it time? CMAJ. 2010;185:1638–42.
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.
- Yu DS, Lee DT, Kwong AN, Thompson DR, Woo J. Living with chronic heart failure: a review of qualitative studies of older people. J Adv Nurs. 2008;61:474-83.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528


