The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Review Article
Medical Management of Obesity
Volume 1, Apr 2012
Suchitra Behl, MD, Seema Gulati, PhD, Swati Bhardwaj, MSc, Divya Parashar, PhD, Anoop Misra, MD; New Delhi, India
Volum 1, April 2012
The prevalence of obesity is increasing worldwide. Obesity is a serious health problem with associated morbidities and increased mortality. A number of diseases including type 2 diabetes mellitus (T2DM), hypertension (HTN), dyslipidemia, coronary heart disease (CHD), stroke, etc. are associated with obesity.Background
Various cultural, social, environmental, and financial factors are implicated in the current rise in prevalence of obesity. Increasing affluence, easy affordability, and adoption of more westernized lifestyle have led to major changes in eating habits. Availability of energy-dense foods including prepackaged foods, increased availability of junk food, increased individual consumption of oil, decreasing physical activity, and stress have led to an increased prevalence of obesity, which is noted to be spread across different socioeconomic classes.
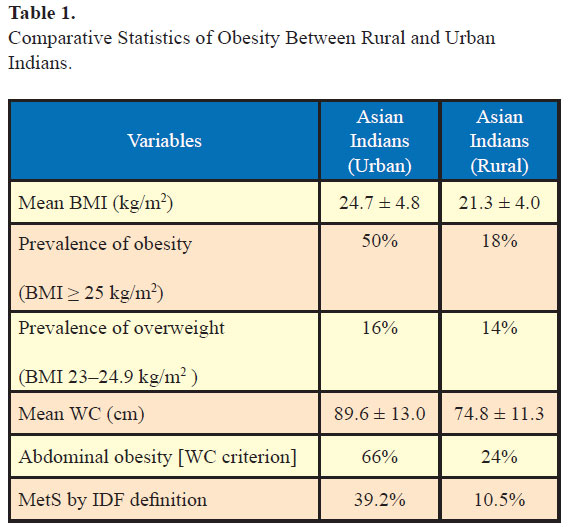
As per a recent study which looked at the prevalence of obesity in urban and rural India, 16% of urban Indians and 14% of rural Indians were overweight and 50% of urban Indians and 18% of rural Indians were obese as per body mass index (BMI) by Asian Indian criteria (1). Further, 66% of urban respondents and 24% of rural respondents had abdominal obesity by waist circumference criteria and this was significantly more in females compared to males (1). Of note, the prevalence of metabolic syndrome as per IDF definition was 39.2% in urban and 10.5% in rural Indians (Table 1). These figures are significant as both obesity and the metabolic syndrome are important risk factors for development of T2DM and CHD. Both are associated with nonalcoholic fatty liver disease and are risk factors for chronic kidney disease. Other associations include polycystic ovarian syndrome, obstructive sleep apnea, hyperuricemia, and gout and various cancers. Depression has been linked with severe obesity especially in younger women (2) .
The importance of recognition and treatment of obesity lies in the health benefits associated with weight loss. There is 16% reduction in risk of diabetes with every kilogram of weight loss (3) as demonstrated in the Diabetes Prevention Program (DPP) study. Moreover in the 10-year follow-up of the participants in the DPP study, the cumulative incidence of diabetes remained lowest in the lifestyle group (4) highlighting the importance of lifestyle interventions and weight loss in the prevention of diabetes.
Various cultural, social, environmental, and financial factors are implicated in the current rise in prevalence of obesity. Increasing affluence, easy affordability, and adoption of more westernized lifestyle have led to major changes in eating habits. Availability of energy-dense foods including prepackaged foods, increased availability of junk food, increased individual consumption of oil, decreasing physical activity, and stress have led to an increased prevalence of obesity, which is noted to be spread across different socioeconomic classes.
As per a recent study which looked at the prevalence of obesity in urban and rural India, 16% of urban Indians and 14% of rural Indians were overweight and 50% of urban Indians and 18% of rural Indians were obese as per body mass index (BMI) by Asian Indian criteria (1). Further, 66% of urban respondents and 24% of rural respondents had abdominal obesity by waist circumference criteria and this was significantly more in females compared to males (1). Of note, the prevalence of metabolic syndrome as per IDF definition was 39.2% in urban and 10.5% in rural Indians (Table 1). These figures are significant as both obesity and the metabolic syndrome are important risk factors for development of T2DM and CHD. Both are associated with nonalcoholic fatty liver disease and are risk factors for chronic kidney disease. Other associations include polycystic ovarian syndrome, obstructive sleep apnea, hyperuricemia, and gout and various cancers. Depression has been linked with severe obesity especially in younger women (2) .
The importance of recognition and treatment of obesity lies in the health benefits associated with weight loss. There is 16% reduction in risk of diabetes with every kilogram of weight loss (3) as demonstrated in the Diabetes Prevention Program (DPP) study. Moreover in the 10-year follow-up of the participants in the DPP study, the cumulative incidence of diabetes remained lowest in the lifestyle group (4) highlighting the importance of lifestyle interventions and weight loss in the prevention of diabetes.

All BMI values in kg/m2
WC, Waist circumference; MetS, metabolic syndrome Adapted from (1).
Definition of obesity for Asian Indians
Definition of obesity for Asian Indians
It has been noted that the prevalence of obesity-related noncommunicable diseases in white Caucasian and Asian Indian populations is similar while obesity prevalence is lower in Asian Indians as per universal BMI cut-off (>30kg/m2). At the same time, the body composition of Asian Indians is different from white Caucasians due to higher truncal subcutaneous, and intra-abdominal adiposity in Asian Indians compared to white Caucasians (5). A WHO Expert Consultation concluded that Asians have high risk of T2DM and cardiovascular disease (CVD) at lower BMI (≤25 kg/m2) and identified potential public health action points [23.0, 27.5, 32.5, and 37.5 kg/m2] along the continuum of BMI (6). Hence the BMI cutoffs and the waist circumference cut-offs should ideally be lower in Asian Indians. In view of these reasons, the Consensus Statement for Diagnosis of Obesity for Asian Indians (7) has recommended that the BMI and waist circumference cut-offs for diagnosis of obesity and abdominal obesity, respectively, are lower than those for white Caucasians (see next section).
Measures of obesity
Screening and diagnosis
Measures of obesity
Body mass index
Body mass index is a practical, easily obtainable, and reproducible measure of obesity. BMI is calculated by dividing body weight in kilogram by height in meter squared. Asian Indians with BMI between 23 and 24.9 kg/m2 are considered overweight and more than or equal to 25 kg/m2 are considered obese (5, 8).
Waist circumference
Waist circumference is another easily obtainable and reproducible measure of abdominal obesity. Waist circumference should be measured just above the iliac crest with a nonstretchable flexible measuring tape at the end of normal expiration. Patient should be in the fasting state and be instructed to stand erect and look straight forward. The observer (physician) should be seated in front of the subject (5). Waist circumference more than 80 cm in females and 90 cm in males (8) predict abdominal obesity in Asian Indians.
Other methods of determining body fat
Bioelectrical impedance analysis (BIA) and dual energy X-ray absorptiometry (DXA) have been used to determine body fat percentage and body composition. These methods are more expensive and available only in large centers. BIA estimates opposition to flow of electric current through body tissues and estimates body fat using an equation. DXA uses X-ray beams of different energy levels to estimate body fat content. Hydrodensitometry and whole body plethysmography use water displacement and air displacement to measure body fat. Computerized Tomography and Magnetic Resonance are other imaging modalities which can determine patterns of body fat distribution.
Etiology
While most cases of obesity are due to imbalance between caloric intake and expenditure coupled with a sedentary lifestyle, other causes of obesity should be looked into.
Genetic
Most cases of obesity are multifactorial. Currently nine loci are recognized which are linked to monogenic obesity and 58 loci have been associated with polygenic obesity (9). Some monogenic causes of obesity identified include congenital leptin deficiency, leptin receptor deficiency, pro-opiomelanocortin (POMC) gene mutations, pro-hormone convertase 1 (PCSK1) mutations, human melanocortin-4 receptor (MC4R) mutations, brain-derived neurotrophic factor (BDNF) variants, and fat mass and obesity associated (FTO) gene mutations (9–17). Some of these gene mutations are more prevalent in certain population groups. Four genes (MC4R, PCSK1, POMC, and BDNF) are involved in both monogenic and polygenic forms of obesity (9). While it has been observed that obesity runs in some families, a shared environment of excessive food intake and low physical activity may be responsible for early manifestation of obesity.
Some inherited genetic syndromes include obesity as a feature, for example, Prader-Willi and Bardet-Biedl. Th disorders are usually evident in childhood.
Some inherited genetic syndromes include obesity as a feature, for example, Prader-Willi and Bardet-Biedl. Th disorders are usually evident in childhood.
Endocrine
Cushing’s syndrome, polycystic ovary syndrome, hypogonadism, hypothyroidism, and growth hormone deficiency should be diagnosed with appropriate hormonal tests when suspected clinically.
Psychosocial factors
Social factors include socioeconomic status and ethnicity. Overeating, binge eating, and high fat diets can lead to obesity. Night eating syndrome is an emerging eating disorder characterized by an ongoing persistent pattern of late night binge eating.
Other factors
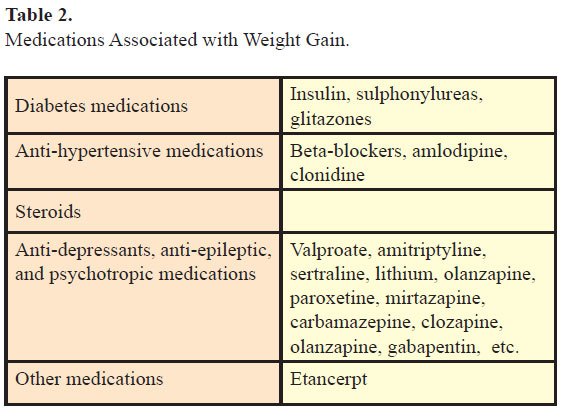
Medications (Table 2), smoking cessation, enforced inactivity, for example, postoperative period, perinatal period, and inactivity related to underlying medical conditions may be associated with weight gain.
Key elements in obtaining history
Components of physical examination

Clinical work-up
Key elements in obtaining history
- Onset, rate, total duration of weight gain
- Dietary recall and patterns of eating
- Previous attempts at weight loss, relapse, and factors associated with weight regain
- List of medications, any weight loss drugs, dietary supplements, over-the-counter drugs, steroid use in the past
- Smoking history, attempts at cessation, and alcohol intake
- History indicative of comorbidities like diabetes mellitus, hypertension, dyslipidemia, heart disease, stroke, sleep apnea
Components of physical examination
A thorough physical examination should be performed looking for markers of metabolic syndrome and other important signs including acanthosis nigricans, xanthalesma, double chin, buffalo hump, tendon xanthoma, gynecomazia, skin tags, arcus, excess supra-clavicular fat pads, thyromegaly, and parotid enlargement.
Biochemical, radiological, and other investigations
A basic metabolic work-up should include fasting lipid panel and fasting blood glucose. When physical examination or history is suggestive serum cortisol and thyroid stimulating hormone (TSH) may be ordered. Investigations directed toward existing comorbidities like electrocardiogram, echocardiogram, polysomnography (PSG), ultrasound of the liver, etc. should be ordered as needed.
Management
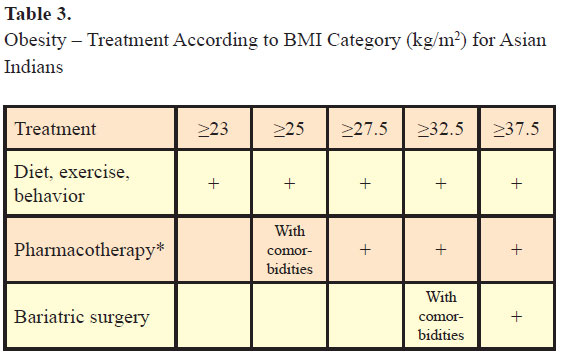
Management involves changes in diet to decrease calorie intake, increase energy expenditure with exercise, drugs, behavioral therapy, and surgical management (see Table 3). A review of long-term weight loss studies in obese adults noted <5 kg weight loss after 2–4 years with dietary/lifestyle changes and 5–10 kg weight loss after 1–2 years with pharmacological therapy (18). Weight loss of 5–10% is associated with reduction in incidence of T2DM (19) and cardiovascular risk factors in patients with cardiovascular risks (18).

*Could also be considered if waist circumference is 10 cm above cut-offs defined for Asian Indians.
Adapted from (8).
Setting targets
A reasonable approach is to discuss various alternatives like dietary changes and medications with the patient and then set goals of weight loss. While the weight loss goal in the DPP study was 7% from the initial body weight, a target of 5–10% weight loss from the initial body weight in the first 6 months may be given.
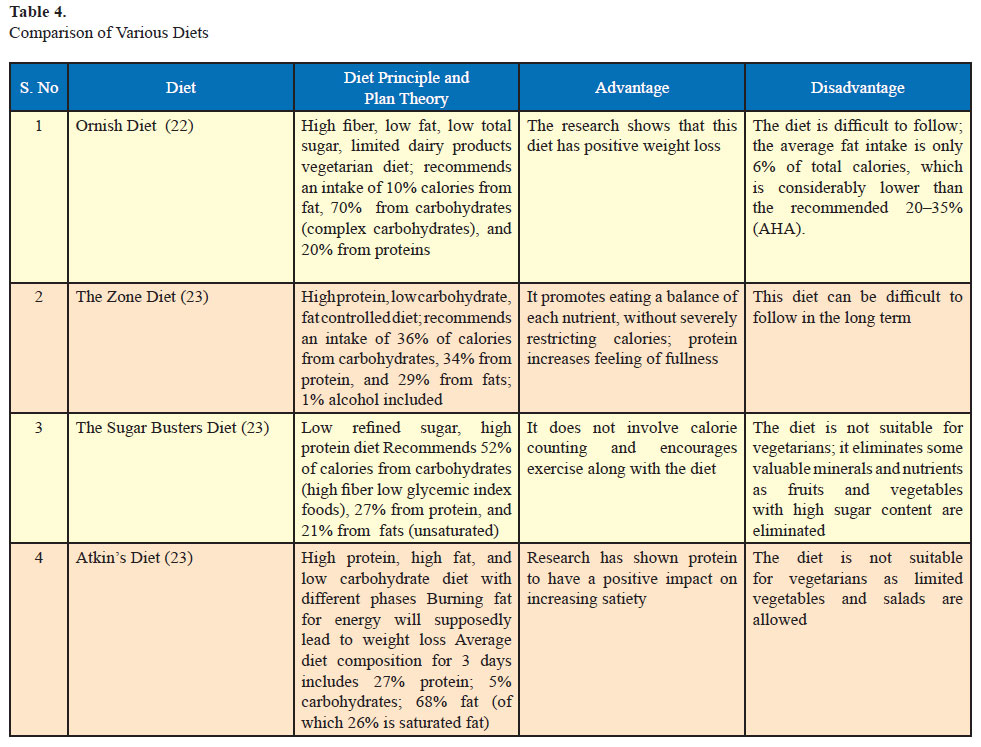
Various diets are available to achieve weight loss. Broadly they may be classified into balanced low calorie, moderate fat low calorie, low fat low calorie, low carbohydrate, and the Mediterranean diets. A comparison of various diets is given in Table 4. A systematic review found that low carbohydrate/high protein (LC/HP) are more effective at 6 months as low fat (LF) diets are as effective, if not more effective, as LF diets in reducing weight at 1 year (20). A vegetarian high protein, low carbohydrate diet for the Indian population is currently under trial by our group. Very low calorie diets (200– 800 kcal/d) produce faster short-term weight loss but are not recommended as there is no difference in long-term weight loss compared to conventional diets (21) and it is difficult to adhere to such diets.
Steps for dietary management are outlined below. For further details on dietary modifications for Asian Indians, please refer to a recent consensus statement (7).
Determination of ideal calorie consumption
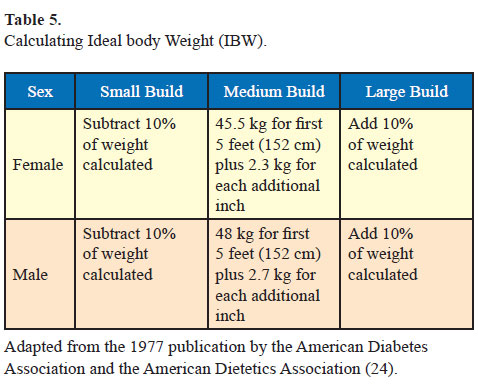
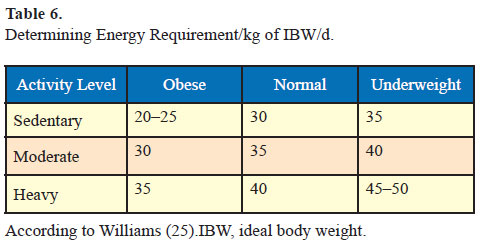
Example: A 165-cm Asian Indian sedentary male with medium body frame has an ideal body weight of 62 kg. If his actual body weight is 82 kg, his BMI is 30.1 kg/m2; he will be classified as obese. His daily calorie requirements will be 1550 kcal/d (62 [IBW] x 25 [energy requirement/ kg IBW/d]). After calculating his daily calorie intake, 300–350 kcal reduction is suggested and a diet plan of about 1200 kcal/d is provided. Exercise schedule which leads to energy expenditure of about 250–300 kcal per day is prescribed. This would result in net daily calorie deficit of 500–600 kcal/d from dietary sources and energy expenditure from exercise. Over a period of 7 days, this should result in 3500 kcal deficit which would result in weight loss of about 500 g per week. Over a period of a month, the patient should be expected to lose about 2 kg with these lifestyle modifications.
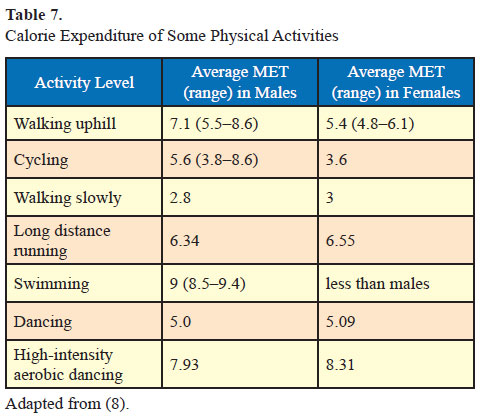
The calorie expenditure of some common physical activities is detailed below (7).
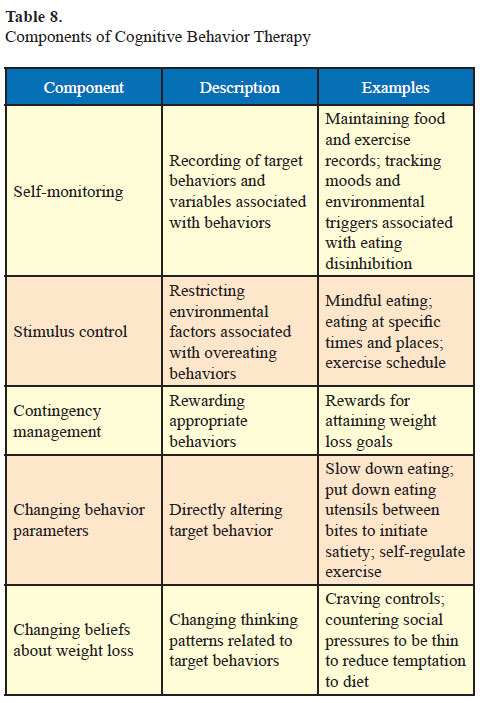
Psychological variables such as anxiety and depression, binge eating, eating disinhibition, mindless eating, general cognitive style, attitude toward food, and lifestyle are important factors to address in obesity. Cognitive behavior therapy focuses on changing the unhealthy patterns of thinking and behaving (29). Components of cognitive behavior therapy are discussed in Table 8.
The holistic treatment approach involves analyses of the factors related to inappropriate eating and exercise such as affective states, personal relationships, irrationalthoughts, family or work stressors. The goal of treatment is to initiate and maintain a process of problem solving to determine whether the removal, reduction, or modification of the causal factors results in more prudent weight management behaviors. Educating the client about the different types of hunger, controlling cravings and promoting mindful eating using behavior modification techniques, and motivating them to adopt a healthier lifestyle are imperative.
While discussing drug therapy, the patient must be made aware that the weight loss associated with medication use is not continuous. After maximum effect is reached weight loss ceases and drug withdrawal may be associated with weight gain in certain cases.

Dietary management
A normal health diet should derive 50–60% of total calorie intake from carbohydrates (preferentially complex carbohydrates), 30% of total calorie intake from fat, and 10–15% from proteins. A minimum of four or five servings per day of fruits and vegetables and 25–40 g/d of fiber are suggested (7). Foods with low glycemic index can be advised.
Various diets are available to achieve weight loss. Broadly they may be classified into balanced low calorie, moderate fat low calorie, low fat low calorie, low carbohydrate, and the Mediterranean diets. A comparison of various diets is given in Table 4. A systematic review found that low carbohydrate/high protein (LC/HP) are more effective at 6 months as low fat (LF) diets are as effective, if not more effective, as LF diets in reducing weight at 1 year (20). A vegetarian high protein, low carbohydrate diet for the Indian population is currently under trial by our group. Very low calorie diets (200– 800 kcal/d) produce faster short-term weight loss but are not recommended as there is no difference in long-term weight loss compared to conventional diets (21) and it is difficult to adhere to such diets.
Steps for dietary management are outlined below. For further details on dietary modifications for Asian Indians, please refer to a recent consensus statement (7).
Determination of ideal calorie consumption
- Calculate the ideal body weight as per Table 5.
- Determine the energy requirement/kg of IBW/day as per Table 6.
- Determine total energy requirement/d as below. Energy requirement in kcal/d= [Ideal body weight] x [energy requirement/kg IBW/d]
- Calculate current calorie intake. The total calorie intake is compared to energy requirement. In most cases calorie intake exceeds requirement. Calorie intake should then be formulated as per ideal body weight and activity factor. This in conjunction with increase in physical activity should result in weight loss. In certain situations, further reduction in calorie intake may be warranted under the supervision of a nutritionist.


Example: A 165-cm Asian Indian sedentary male with medium body frame has an ideal body weight of 62 kg. If his actual body weight is 82 kg, his BMI is 30.1 kg/m2; he will be classified as obese. His daily calorie requirements will be 1550 kcal/d (62 [IBW] x 25 [energy requirement/ kg IBW/d]). After calculating his daily calorie intake, 300–350 kcal reduction is suggested and a diet plan of about 1200 kcal/d is provided. Exercise schedule which leads to energy expenditure of about 250–300 kcal per day is prescribed. This would result in net daily calorie deficit of 500–600 kcal/d from dietary sources and energy expenditure from exercise. Over a period of 7 days, this should result in 3500 kcal deficit which would result in weight loss of about 500 g per week. Over a period of a month, the patient should be expected to lose about 2 kg with these lifestyle modifications.
Exercise therapy
Physical activity combined with energy restriction will increase weight loss (26) and an exercise prescription should be formulated keeping in mind current activity level (Table 7).
Physical activity combined with energy restriction will increase weight loss (26) and an exercise prescription should be formulated keeping in mind current activity level (Table 7).
- Patients who are physically inactive should be advised to start low-intensity exercise and to gradually increase exercise intensity. For example a sedentary patient can be advised walking with gradual increase in intensity to brisk walking, then jogging, and further to running. Patients with chronic medical conditions should consult their doctor before starting any exercise regimen (8).
- For patients with obesity daily 60 minutes of physical activity (420 min/week) is recommended (8). In order to achieve weight loss, this physical activity should include 300 min/week of moderate-intensity physical activity or 150 min/week of vigorousintensity physical activity. Brisk walking, jogging, cycling, swimming, or muscles strengthening activity like resistance exercises are some options of moderate intensity and running, dancing, football, badminton can be suggested as vigorous-intensity activities (8). Moderate intensity between 150 and 250 min/week will improve weight loss in studies that used moderate diet restriction but not in studies that used severe diet restriction (26). For detailed discussion of physical activity please refer to the recent consensus statement (8).
- Studies have shown that after weight loss, weight maintenance is improved with physical activity of more than 250 min/week (26).
The calorie expenditure of some common physical activities is detailed below (7).

Behavioral interventions
Behavioral therapy aims to modify maladaptive eating patterns and physical activity and is an adjunct to dietary counseling, exercise, and medication (27, 28). Behavioral treatment both individually and in group sessions helps in both weight loss and weight maintenance.
Psychological variables such as anxiety and depression, binge eating, eating disinhibition, mindless eating, general cognitive style, attitude toward food, and lifestyle are important factors to address in obesity. Cognitive behavior therapy focuses on changing the unhealthy patterns of thinking and behaving (29). Components of cognitive behavior therapy are discussed in Table 8.

The holistic treatment approach involves analyses of the factors related to inappropriate eating and exercise such as affective states, personal relationships, irrationalthoughts, family or work stressors. The goal of treatment is to initiate and maintain a process of problem solving to determine whether the removal, reduction, or modification of the causal factors results in more prudent weight management behaviors. Educating the client about the different types of hunger, controlling cravings and promoting mindful eating using behavior modification techniques, and motivating them to adopt a healthier lifestyle are imperative.

Medications Associated with Weight Loss
There are few drugs available for weight loss although research is ongoing and there may be more options in the future. A number of drugs have been withdrawn from the market including dinitrophenol, aminorex, fenfluramines, phenylpropanolamine, rimonabant, and sibutramine. Sibutramine blocks norepinephrine and serotonin reuptake. It is associated with early satiety. It was withdrawn for use due to increased risk of nonfatal myocardial infarction and nonfatal stroke.
While discussing drug therapy, the patient must be made aware that the weight loss associated with medication use is not continuous. After maximum effect is reached weight loss ceases and drug withdrawal may be associated with weight gain in certain cases.
Orlistat
Orlistat is the only drug approved for long-term treatment of obesity and the only drug approved for treatment of obesity in children. Orlistat acts by inhibiting pancreatic lipase and preventing the digestion and absorption of dietary fat. As a result of these actions fecal fat excretion is increased.
In the European Multicentre Orlistat Study Group patients in the orlistat group lost more weight compared to placebo group (10.2% [10.3 kg] vs 6.1% [6.1 kg]) in the first year. In the second year, patients who continued on orlistat regained half as much weight as those switched to placebo (30). It has low (less than 1%) systemic absorption. This drug has been studied for upto 4 years of continued use (31). In addition, orlistat has other beneficial effects in lowering blood pressure and decreasing the risk of development of diabetes in patients with impaired glucose tolerance at baseline (31). In trials, the total cholesterol, low-density lipoprotein (LDL) cholesterol, LDL/high-density lipoprotein ratio, and concentrations of glucose and insulin decreased more in the orlistat group than in the placebo group (30).
In the European Multicentre Orlistat Study Group patients in the orlistat group lost more weight compared to placebo group (10.2% [10.3 kg] vs 6.1% [6.1 kg]) in the first year. In the second year, patients who continued on orlistat regained half as much weight as those switched to placebo (30). It has low (less than 1%) systemic absorption. This drug has been studied for upto 4 years of continued use (31). In addition, orlistat has other beneficial effects in lowering blood pressure and decreasing the risk of development of diabetes in patients with impaired glucose tolerance at baseline (31). In trials, the total cholesterol, low-density lipoprotein (LDL) cholesterol, LDL/high-density lipoprotein ratio, and concentrations of glucose and insulin decreased more in the orlistat group than in the placebo group (30).
Adverse drug reactions that should be discussed with the patient include gastrointestinal side-effects like cramps, fecal urgency, oily spotting/evacuation, and flatus. Patients stopping orlistat may regain weight (30). Orlistat is available as 60 and 120 mg doses. Treatment may be started at 60 mg dose to be increased to 120 mg in 2 weeks. Orlistat may cause decrease in absorption of fat soluble vitamins (A, D, E, and beta-carotene] and supplementation of these vitamins may be advised.
Metformin
Metformin is widely used for treatment of diabetes, impaired glucose tolerance, and polycystic ovarian syndrome. It has been shown to cause weight loss (1–2kg) in patients with obesity and the metabolic syndrome (32). In the DPP study, patients with impaired glucose tolerance in the metformin group lost a mean of 2.5 kg which was maintained in the 10 year follow-up study (4, 19).These patients also had lower incidence of diabetes as compared to the placebo group, while the lowest incidence of diabetes was observed in the lifestyle intervention group (4).
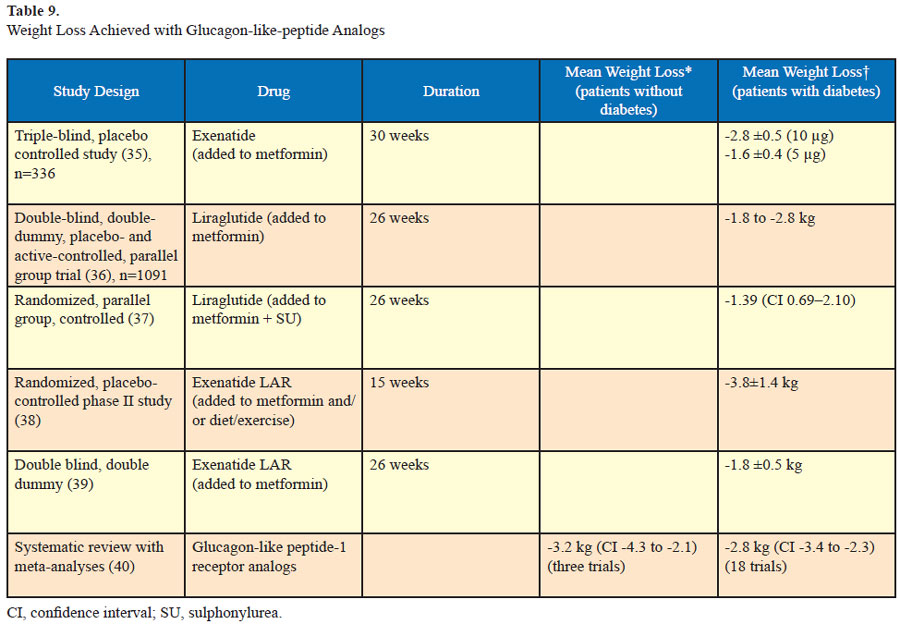
Glucagon-like-peptide 1 analogs
Long-acting glucagon-like peptides include exenatide, liraglutide, and exenatide once weekly (Table 9). These incretin peptides are approved for treatment of diabetes. They increase glucose-dependant insulin secretion from the pancreas, insulin sensitivity in pancreatic alpha and beta cells¸ satiety in the brain, and decrease gastric emptying. Exenatide has been found to cause dosedependent weight loss in trials of patients with diabetes. Similarly weight loss has also been reported in trials of liraglutide in diabetic patients as well as in a trial of nondiabetic patients. These drugs are associated with satiety and decreased gastric emptying. Currently they are off label for the treatment of obesity in nondiabetic patients.
In a 26-week trial which compared liraglutide (1.8k mg daily) with exenatide (10 μg twice daily) in diabetic patients similar weight loss was noted (2.8–3.2 kg). Adverse drug reactions included nausea, vomiting, and diarrhea in both groups (33). In the DURATION-5 trial which compared exenatide once weekly to the twice daily formulation, similar weight loss was noted in both groups (-2.3 ±0.4 kg and -1.4 ± 0.4 kg). Mild to moderate nausea was the most frequent adverse effect and it was less common with exenatide once weekly compared to exenatide twice daily (14% vs 35%) (34).
Conclusion
In a 26-week trial which compared liraglutide (1.8k mg daily) with exenatide (10 μg twice daily) in diabetic patients similar weight loss was noted (2.8–3.2 kg). Adverse drug reactions included nausea, vomiting, and diarrhea in both groups (33). In the DURATION-5 trial which compared exenatide once weekly to the twice daily formulation, similar weight loss was noted in both groups (-2.3 ±0.4 kg and -1.4 ± 0.4 kg). Mild to moderate nausea was the most frequent adverse effect and it was less common with exenatide once weekly compared to exenatide twice daily (14% vs 35%) (34).
Phentermine
Phentermine, a psycho stimulant drug of the phenethylamine class, functions as an appetite suppressant. Similar to amphetamines, it can cause tachycardia, elevated blood pressure, palpitations, restlessness, and insomnia. It can cause psychological dependence. Rebound weight gain can occur once drug tolerance develops. It is approved by US FDA for shortterm use up to 12 weeks.
Other drugs
Other drugs
- Diethylpropion and phenylpropanolamine are norepinephrine releasing agents that are appetite suppressants. Phenylpropanolamine is another norepinephrine releasing agent. Both drugs are banned for use in India.
- Bupropion and fluoxetine are associated with weight loss but neither is approved by US FDA for treatment of obesity.
- Tesofenasine, which was initially developed as a medication for Parkinson’s disease, has been noted to produce weight loss. It has been studied in a trial of obese patients where weight loss was confirmed but further trials are awaited regarding safety and side-effects.
- A phentermine–topiramate combination pill is pending FDA approval. Topiramate used as antiepileptic has been associated with weight loss. Early studies have shown up to 10% weight loss after a year of use of this combination pill. Sideeffects include lowering of blood pressure and raising heart rate. Topiramate is associated with birth defects (cleft palate) and this must be kept in mind while prescribing.
Conclusion
Recent years have witnessed an increasing prevalence of obesity which is spread across different age groups and noted in both rural and urban areas. This increasing prevalence of obesity and its associated medical problems necessitates that all patients visiting a medical practitioner should be screened for obesity. Asian Indians with BMI between 23 and 24.9 kg/m2 should be diagnosed as overweight and those with BMI more than or equal to 25 kg/m2 should be diagnosed as obese.
Patients with waist circumference more than 80 cm in females and 90 cm in males should be diagnosed with abdominal obesity. BIA and DXA which estimate body
fat may be ordered at centers where they are available.
Most cases of obesity are due to increased caloric intake but other causes of obesity should be ruled out. Patients with obesity often have comorbidities like T2DM, HTN, CHD, dyslipidemia, etc., and they should be looked for and appropriately managed. All patients who are diagnosed as being overweight or obese should be prescribed diet, exercise, and behavior therapy. Pharmacotherapy should be given to patients with BMI of 27.5 kg/m2 or above and to patients with BMI between 25 and 27.5 kg/m2 in the presence of comorbidities. Bariatric surgery can be recommended to patients with BMI of 37.5 kg/m2 or above and to patients with BMI between 32 and 37.5 kg/m2 in the presence of omorbidities.
References
Most cases of obesity are due to increased caloric intake but other causes of obesity should be ruled out. Patients with obesity often have comorbidities like T2DM, HTN, CHD, dyslipidemia, etc., and they should be looked for and appropriately managed. All patients who are diagnosed as being overweight or obese should be prescribed diet, exercise, and behavior therapy. Pharmacotherapy should be given to patients with BMI of 27.5 kg/m2 or above and to patients with BMI between 25 and 27.5 kg/m2 in the presence of comorbidities. Bariatric surgery can be recommended to patients with BMI of 37.5 kg/m2 or above and to patients with BMI between 32 and 37.5 kg/m2 in the presence of omorbidities.
References
- Vinch CS, Hill JC, Logsetty G, Tighe DA, Meyer TE, Aurigemma GP. Paradoxical changes in brain natriuretic peptide levels and loading conditions after intravenous conscious sedation. J Am Soc Echocardiogr. 2004; 17(11):1191–6.
- Dixon JB, Dixon ME, O’Brien PE. Depression in association with severe obesity: changes with weight loss. Arch Intern Med. 2003; 163(17):2058–65.
- Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006; 29(9):2102–7.
- Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009; 374(9702):1677–86.
- Ortega O, Gallar P, Muñoz M, Rodríguez I, Carreño A, Ortiz M, Molina A, Oliet A, Lozano L, Vigil A. Association between C-reactive protein levels and N-terminal pro-B-type natriuretic peptide in pre-dialysis patients. Nephron Clin Pract. 2004; 97(4):c125–30.
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363(9403):157–63.
- Misra A, Sharma R, Gulati S, Joshi SR, Sharma V, Ghafoorunissa, Ibrahim A, Joshi S, Laxmaiah A, Kurpad A, Raj RK, Mohan V, Chandalia H, Krishnaswamy K, Boindala S, Gopalan S, Bhattiprolu SK, Modi S, Vikram NK, Makkar BM, Mathur M, Dey S, Vasudevan S, Gupta SP, Puri S, Joshi P, Khanna K, Mathur P, Krishnaswamy S, Madan J, Karmarkar M, Seth V, Passi SJ, Chadha D, Bhardwaj S; National Dietary Guidelines Consensus Group. Consensus dietary guidelines for healthy living and prevention of obesity, the metabolic syndrome, diabetes, and related disorders in Asian Indians. Diabetes Technol Ther. 2011; 13(6):683–94.
- Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, Joshi SR, Sadikot S, Gupta R, Gulati S, Munjal YP; Concensus Group.. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009; 57:163–70.
- Choquet H, Meyre D. Genetics of obesity: what have we Learned? Curr Genomics. 2011; 12(3):169–79.
- Farooqi IS, O’Rahilly S. Monogenic human obesity syndromes. Recent Prog Horm Res. 2004; 59:409–24.
- Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O’Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004; 101(13):4531–6.
- Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O’Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007; 356(3):237–47.
- Benzinou M, Creemers JW, Choquet H, Lobbens S, Dina C, Durand E, Guerardel A, Boutin P, Jouret B, Heude B, Balkau B, Tichet J, Marre M, Potoczna N, Horber F, Le Stunff C, Czernichow S, Sandbaek A, Lauritzen T, Borch-Johnsen K, Andersen G, Kiess W, Körner A, Kovacs P, Jacobson P, Carlsson LM, Walley AJ, Jørgensen T, Hansen T, Pedersen O, Meyre D, Froguel P. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat Genet. 2008; 40(8):943–5.
- Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, et al (258 Collaborators). Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008; 40(6):768–75.
- El-Gharbawy AH, Adler-Wailes DC, Mirch MC, Theim KR, Ranzenhofer L, Tanofsky-Kraff M, Yanovski JA. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. J Clin Endocrinol Metab. 2006; 91(9):3548–52.
- Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008; 359(9):918–27.
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007; 316(5826):889–94.
- Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond). 2005; 29(10): 1153–67.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346(6):393–403.
- Hession M, Rolland C, Kulkarni U, Wise A, Broom J. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/ low-calorie diets in the management of obesity and its comorbidities Obes Rev. 2009; 10(1):36–50.
- Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring). 2006; 14(8):1283–93.
- Shikany JM, Thomas SE, Henson CS, Redden DT, Heimburger DC. Glycemic index and glycemic load of popular weight-loss diets. MedGenMed. 2006; 8(1):22.
- St Jeor ST, Howard BV, Prewitt TE, Bovee V, Bazzarre T, Eckel RH. Dietary protein and weight reduction: a statement for healthcare professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2001; 104(15):1869–74.
- Feldman DS, Ikonomidis JS, Uber WE, Van Bakel AB, Pereira NL, Crumbley AJ 3rd, Tann SM.. Human B-natriuretic peptide improves hemodynamics and renal function in heart transplant patients immediately after surgery. J Card Fail. 2004; 10(4):292–6.
- Mottram PM, Haluska BA, Marwick TH. Response of B-type natriuretic peptide to exercise in hypertensive patients with suspected diastolic heart failure: correlation with cardiac function, hemodynamics, and workload. Am Heart J. 2004; 148(2):365–70.
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009; 41(2):459–71
- Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, Hesson LA, Osei SY, Kaplan R, Stunkard AJ. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005; 353(20):2111–20.
- Leblanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011; 155(7):434–47.
- Van Buren DJ, Sinton MM. Psychological aspects of weight loss and weight maintenance. J Am Diet Assoc. 2009; 109(12):1994–6. 30.
- Sjöström L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, Krempf M. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998; 352(9123):167–72
- Van Buren DJ, Sinton MM. Psychological aspects of weight loss and weight maintenance. J Am Diet Assoc. 2009; 109(12):1994–6. 30. Sjöström L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, Krempf M. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998; 352(9123):167–72
- Fontbonne A, Charles MA, Juhan-Vague I, Bard JM, André P, Isnard F, Cohen JM, Grandmottet P, Vague P, Safar ME, Eschwège E. The effect of metformin on the metabolic abnormalities associated with upperbody fat distribution. BIGPRO Study Group. Diabetes Care. 1996; 19(9):920–6.
- Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L; LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009; 374(9683):39–47.
- Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L; DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, openlabel, non-inferiority study. Lancet. 2008; 372(9645):1240–50.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005; 28(5):1092–100.
- Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, Düring M, Matthews DR; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009; 32(1):84–90.
- Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simó R; Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009; 52(10):2046–55.
- Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, Taylor K. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007; 30(6):1487–93.
- Wysham C, Bergenstal R, Malloy J, Yan P, Walsh B, Malone J, Taylor K. DURATION-2: efficacy and safety of switching from maximum daily sitagliptin or pioglitazone to once-weekly exenatide. Diabet Med. 2011; 28(6):705–14.
- Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012; 344:d7771.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528


