The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Brief Report
Echocardiographic Evaluation of Right Ventricular Function in Healthy Full-term Neonates during First Week of Life
Volume 2, Oct 2013
S. R. Mittal, DM (Cardiology), Ajmer, Rajasthan, India
J Clin Prev Cardiol. 2013;2(4):195-201
Evaluation of right ventricular function is important in neonates with congenital heart disease (1). Most of the neonates with suspected heart disease undergo echocardiographic evaluation during first week of life. Echocardiographers mostly look at structural defects. Importance of ventricular function in this age group is not widely appreciated. With similar structural defect, prognosis and postoperative results are likely to depend on myocardial function. There is no literature about detailed echocardiographic evaluation of right ventricular function in this age group (2–9). We, therefore, performed detailed echocardiographic evaluation of right ventricular function in healthy full-term neonates during first week of life to find normal pattern in this age group.Material and Methods
The study was approved by institutional ethical committee. Informed consent was obtained from all parents. Twenty-five healthy full-term neonates were studied during first week of life. They had no cardiorespiratory or systemic disease. They were not receiving any drugs and were breathing room air. They did not have any abnormality on two-dimensional or Doppler echocardiography. Sixteen were males and nine were females. Mean age was 4.8±2.8 days. Mean heart rate was 121±17/min. Mean weight was 2.8±0.7 kg.
Exclusion Criteria
Neonates with history of asphyxia were excluded as even mild asphyxia can affect myocardial function (10). Neonates with patent ductus arteriosus were excluded to avoid any possible impact of increased pulmonary flow and left ventricular volume overload on right ventricular function. Neonates with any atrial septal defect or those with any evidence of pulmonary artery hypertension were excluded to avoid effect of right ventricular volume or pressure overload on right ventricular function. Neonates with any valvular lesion were excluded. Neonates with any rhythm disorder or conduction defect were excluded. Neonates of diabetic mothers were excluded as such neonates are known to have myocardial dysfunction (11). Preterm neonates, those with intrauterine growth retardation and those with chromosomal abnormalities/syndromes were also excluded as such neonates are likely to have cardiac function abnormalities (12,13).
Due to fast heart rate and respiratory rate, effect of single respiratory cycle is much less in neonates (6). Further, any phase lag between respiration and ventricular filling is difficult to discern at fast heart and respiratory rates (6). Therefore, average of consecutive five cardiac cycles was taken for each parameter to minimize the effect of respiration (7). Data are presented as mean±SD.

Echocardiography
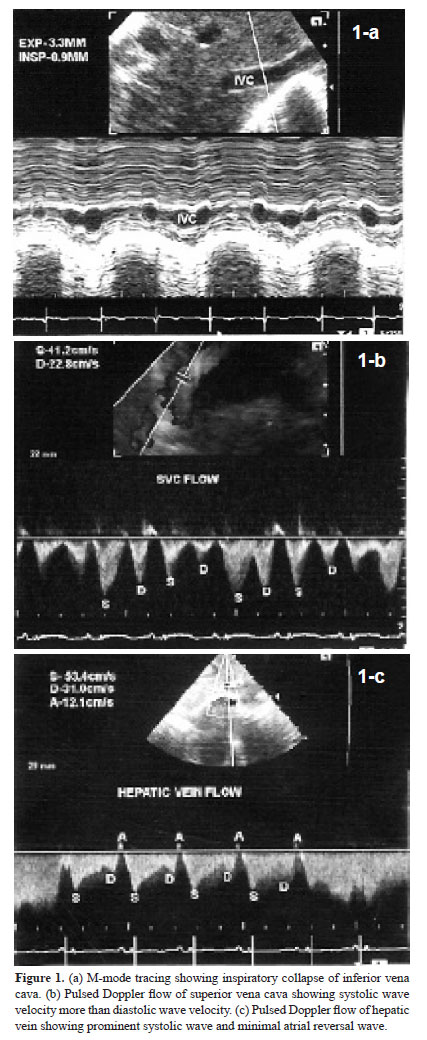
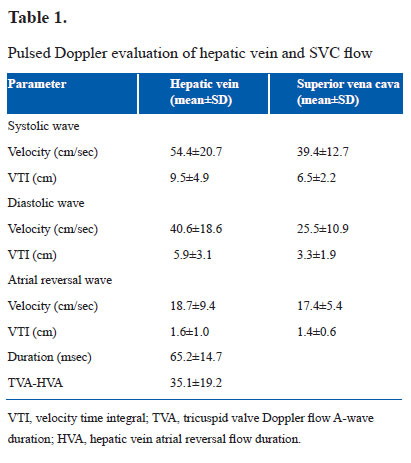
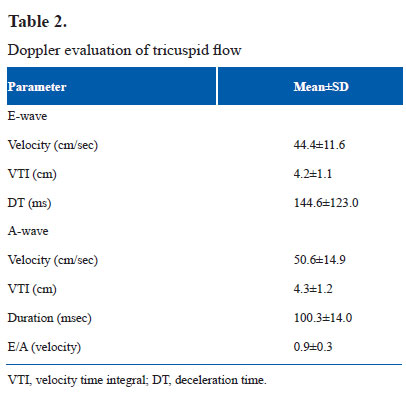
It was performed on Siemens Acuson X300 with facility for tissue Doppler imaging. Phased array transducer with frequency of 4–8 MHz was used. Dimensions of inferior vena cava (IVC) and flow pattern in hepatic vein (HV) and superior vena cava (SVC) were evaluated in supine position. Rest of the examination was performed in slight right anterior oblique position. Dimensions of IVC were measured at a point distal to the joining of HV. M-mode cursor was used. Maximum expiratory and minimum inspiratory dimensions were measured and percentage of inspiratory collapse was calculated (Fig. 1a). SVC flow pattern was recorded by placing transducer in right supraclavicular region (14). Representative tracing is shown in Figure 1b. HV flow pattern was recorded from subcostal long axis view with sample volume in right superior HV just proximal to its connection to IVC (14). Representative tracing is shown in Figure 1c. Velocity and velocity time integral of systolic wave, diastolic wave and atrial reversal wave were analyzed in SVC and HV flow. Tricuspid flow A-wave duration minus HV flow A-wave reversal duration was calculated.
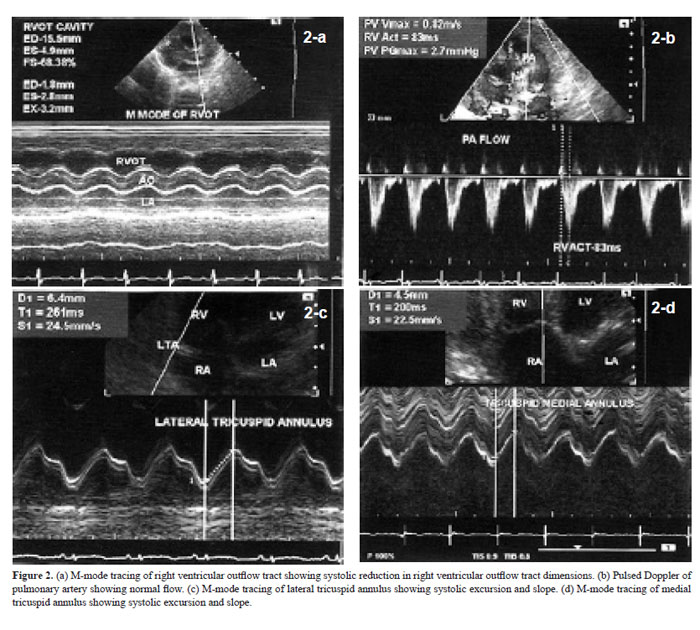
Right ventricular outflow tract (RVOT) dimensions were recorded from left parasternal short axis view at the level of aortic root (15). M-mode cursor was used. RVOT anterior wall thickness and cavity dimensions were measured in end-diastole and end-systole (Fig. 2a). Fractional shortening was calculated. Pulsed Doppler evaluation of pulmonary artery flow was performed in left parasternal short axis view at the level of aortic
root. Sample volume was kept just distal to pulmonary valve. Peak velocity and acceleration time were analyzed (Fig. 2b).
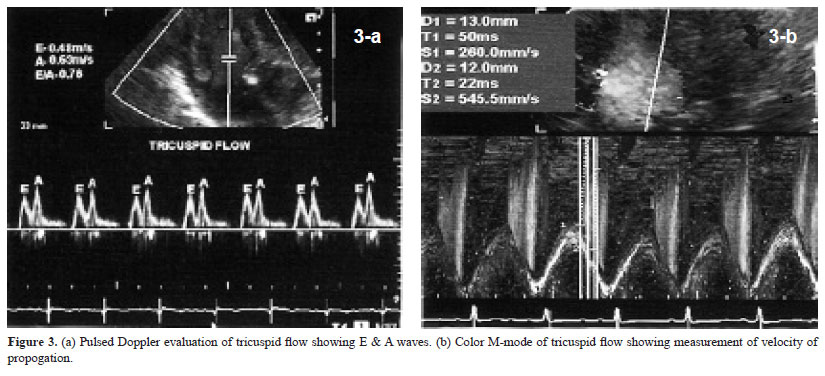
Tricuspid annulus plane systolic excursion was measured using M-mode cursor in apical four-chamber view (16). Lateral and septal parts of annulus were evaluated (Fig. 2c & d). Systolic excursion and slope were analyzed. Tricuspid flow velocities were recorded by pulsed Doppler method. Sample volume was placed at the level of tip of tricuspid leaflets in diastole. Early (E) and late (A) diastolic velocities were recorded
(Fig. 3a). Velocity time integral (VTI) of E- and A-wave were analyzed. E/A ratio was calculated. Deceleration time of E-wave and duration of A-wave were analyzed. When A-wave started before end of E-wave, deceleration time of E-wave was calculated by extrapolation of initial slope to base line. Tricuspid flow propogation velocity (Vp) was recorded in the same view using color Doppler and keeping M-mode cursor in the tricuspid flow. Slopes of early and late diastolic flow were measured (Fig. 3b). Tissue Doppler imaging was also performed in apical four-chamber view (9). Sample volume was kept over medial and lateral part of tricuspid annulus (Fig. 4a & b). Ultrasound beam was kept perpendicular to the plane of the annulus to minimize angle of incidence. Systolic velocity (Sa), early diastolic velocity (Ea), late diastolic velocity (Aa), their VTI, isovolumic contraction time (IVCT), isovolumic relaxation time (IVRT) and ejection time were analyzed and myocardial performance index (MPI) was calculated.
Due to fast heart rate and respiratory rate, effect of single respiratory cycle is much less in neonates (6). Further, any phase lag between respiration and ventricular filling is difficult to discern at fast heart and respiratory rates (6). Therefore, average of consecutive five cardiac cycles was taken for each parameter to minimize the effect of respiration (7). Data are presented as mean±SD.



Results
IVC dimension was 4.4±1.0 mm in expiration and 1.8±0.6 mm in inspiration. Inspiratory collapse was 66.5±13.5%. HV size was 4.6±1.1 mm. HV and SVC flow patterns are shown in Table 1. Systolic wave velocity was more than diastolic wave velocity. Atrial reversal wave had minimum velocity and VTI. Velocities were more in HV than in SVC. Tricuspid flow A-wave duration was more than duration of atrial reversal wave in HV. RVOT anterior wall thickness was 2.2±0.5 mm in end diastole and 3.2±0.8 mm in end systole. Systolic excursion was 3.9±0.8 mm. RVOT cavity dimensions were 10.8±1.9 mm in end diastole and 4.0±1.0 mm in end systole. Fractional shortening was 63.1±6.7%. Pulmonary artery flow peak velocity was 0.7±0.2 cm/sec. Acceleration time was 88.5±17.6 msec. Medial tricuspid annulus plane systolic excursion was 6.1±1.0 mm with a slope of 27.6±6.2 cm/sec. Lateral tricuspid annulus plane systolic excursion was 8.3±1.6 mm with a slope of 37.1±9.0 cm/sec. Pulsed Doppler parameters of tricuspid flow are shown in Table 2. A wave velocity and VTI were more than respective values of E-wave. E/A ratio was less than 1. Velocity of propogation of early tricuspid flow was 44.5±11.9 cm/sec. Vp of late tricuspid flow was 211.1±105.1 cm/sec. E/Vp was 3.2±1.6. Slope of late diastolic flow was rapid than slope of early diastolic flow. Tissue Doppler imaging velocities of medial and lateral part of tricuspid annulus are shown in Table 3. All velocities were more along lateral part of tricuspid annulus. Late diastolic velocity (Aa) was more than early diastolic velocity (Ea).
Discussion
IVC showed inspiratory collapse of more than 50%. This reflects normal inspiratory fall in right ventricular and right atrial pressure (17,18).There is no previous literature about this parameter in neonates. In SVC and HV flow, systolic velocity was more than diastolic velocity with small reversal wave during atrial contraction. This suggests normal filling pattern of right atrium with greater filling in systole than in diastole (19,20). Flow velocities were greater in HV than in SVC. This could be because of closer proximity of HV to right atrium and also because of relatively less compliance of HV (21). Tricuspid flow A-wave duration was more than the duration of atrial reversal wave in HV. There is no previous literature on this parameter. This finding correlates with greater A-wave duration of mitral flow than duration of atrial reversal flow in pulmonary vein (PVa) in normal persons (22).
Evaluation of RVOT revealed good systolic thickening of anterior wall and fractional shortening of more than 50%. These findings are considered suggestive of normal RV systolic function (15). Pulmonary artery flow peak velocity was normal. Pulmonary flow acceleration time is the time interval between beginning of the flow and its peak velocity (23). It was slightly lower in neonates. It could be due to increased afterload caused by physiological pulmonary hypertension. Tricuspid annular excursion reflects longitudinal shortening of the right ventricle (24). Greater excursion of lateral part of the annulus is because the lateral wall of right ventricle is rich in longitudinal fibers. Interventricular septum has more of circumferencial fibers (16,25). Koestenberger et al. (26) evaluated lateral tricuspid annulus plane systolic excursion in infants, children and adolescents. They reported a mean value of 0.91 cm for lateral tricuspid annulus in neonates as a whole (0–30 days). Our observation during first week of life is 0.8±0.2 cm. Slight difference could be due to the effect of maturation with advancing age (7, 11). There is no literature about systolic excursion of medial tricuspid annulus in neonates. We observed a relatively lower value 0.6±0.1 cm for medial tricuspid annulus. This is understable because interventricular septum has less longitudinal fibers (25).
Doppler evaluation of tricuspid flow revealed that late diastolic flow (A) velocity was more than early diastolic (E) flow velocity. E/A ratio was less than 1. Increased A-wave velocity suggests impaired relaxation of right ventricle resulting in forceful right atrial contraction. This finding is in agreement with observation of previous workers (6–9). These observations suggest impaired relaxation of right ventricle in first week of life. This could be because of only partial regression of right ventricular dominance of fetal circulation. Velocity of propogation of late diastolic tricuspid flow was more than that of early diastolic flow suggesting impaired relaxation with rapid filling of RV during right atrial contraction.
Tissue Doppler imaging velocities revealed that all velocities were more along lateral part of tricuspid annulus. Late diastolic velocity (Aa) was more than early diastolic velocity (Ea). Previous studies on lateral tricuspid annulus in neonates had similar observations (9,27). These observations also suggest impaired relaxation of right ventricle during neonatal period. Systolic velocity (Sa) observed by us (6.4±1.0 cm/sec) is similar to values observed by Mori et al. (9) (6.2±1.1 cm/sec) and Alp et al. (6.5±1.4 cm/sec) (27) in neonates.
Ea/Aa ratio (0.9±0.2) and E/Ea ratio (7.1±2.3) of lateral tricuspid annulus are similar to values observed by Alp et al. on first day of life (Ea/Aa 0.72±0.14, E/Ea 7.9±1.6) (27). There is no literature about tissue Doppler imaging velocities of medial tricuspid annulus in neonates. Tissue Doppler imaging of medial tricuspid annulus is also important in evaluation of right ventricular function (28).We observed that Ea/Aa ratio of medial as well as lateral tricuspid annulus was less than 1. This finding also supports impaired relaxation of right ventricle in early neonatal period. We observed that IVRT of lateral tricuspid annulus was 45.3±14.9 msec in neonates. Right ventricular isovolumic relaxation time correlates with pulmonary artery pressure (29). IVCT in our subjects was 44.8±13.2 msec. There is no literature on this parameter in neonates. IVCT corelates with rate of systolic shortening of RV (30). Right ventricular MPI (Tei index) was 0.42±0.13 in our subjects. This could be due to prolonged IVRT. Tei index gives an overall impression about systolic and diastolic performance of RV(31).
Conclusion
At present, there is paucity of data on normal values of various echocardiographic parameters of right ventricular systolic and diastolic function in healthy neonates during first week of life. Right ventricle shows impaired relaxation pattern during this period. This could be due to only partial regression of right ventricular dominance of fetal circulation. Our data provide preliminary information on this topic.
Limitations
Sample size is small. This was because we strictly followed exclusion criteria. Getting well-informed written concent from parents of healthy asymptomatic neonates was another difficulty. Study of larger number of neonates can priovide more information and reference values. Long-term follow-up of same neonates can provide more information about progressive changes in right ventricular function. Comparison with neonates with right ventricular overload, their long-term follow-up and post-operative results is needed to find clinical utility of our observations.
Source of Funding
Nil
Conflict of Interest
Nil
References
- Tulevski II, Bodge-Khatami A, Groenink M, van der Wall EE, Romkes H, Mulder JM. Right ventricular function in congenital heart disease: noninvasive quantitative parameters for clinical follow up. Cardiol Young. 2003;13:397–403.
- Harda K, Orino T, Yasuoka K, Tamura M, Takada G. Tissue Doppler imaging of left and right ventricles in normal children. Tohoku J Exp Med. 2000;191:21–9.
- Frommelt PC, Ballweg JA, Whitstone BN, Frommett MA. Usefulness of Doppler tissue imaging analysis of tricuspid annular motion for determination of right ventricular function in normal infants and children. Am J Cardiol. 2002;89:610–3.
- Borzoee M, Kheirandish Z. Doppler derived myocardial performance index in healthy children in Shiraz. Iran J Med Sci. 2004;29:85–9.
- Eidem BW, Mc Mahon CJ, Cohen RR, et al. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17:212–21.
- Riggs TW, Rodriguez R, Snider AR, Batton D, Poltac J, Sharp EZ. Doppler echocardiographic evaluation of right and left ventricular diastolic function in normal neonates. J Am Coll Cardiol. 1989;13:700–5.
- Ichihashi K, Ewert P, Welmitz G, Lange PE. Change in cardiac diastolic function in neonates. Heart Vessels. 1997;12:216–20.
- Harda K, Shiota T, Takahashi Y, Suzuki T, Tamura M, Takada G. Right ventricular diastolic filling in the first day of life. Tohoku J Exp Med. 1994;172:227–35.
- Mori K, Nakagawa R, Nii M, et al. Pulsed wave Doppler tissue echocardiography assessment of the long axis function of the right and left ventricles during the early neonatal period. Heart. 2004;90:175–80.
- Ichihashi K, Yada Y, Takahashi N, Hanama Y, Momoi M. Utility of Doppler derived index combining systolic and diastolic performance (Tei index) for detecting hypoxic cardiac damage in new born. J Perinat Med. 2005;33:549–52.
- Tsutsumi T, Ishil M, Eto G, Hota M, Kata H. Serial evaluation of myocardial performance in fetuses and neonates using a new Doppler index. Pediatr Int .1999;41:722–7.
- Harda K, Rice MJ, Shiato T, et al. Gestational age and growth related alterations in fetal right and left ventricular diastolic filling patterns. Am J Cardiol. 1997;79:173–7.
- Rizzo G, Arduini D. Fetal cardiac functions in intrauterine growth retardation. Am J Obstet Gynecol. 1991;165:876–82.
- Appleton CP, Hatle LK, Popp RL. Superior vena cava and hepatic vein Doppler echocardiography in healthy adults. J Am Coll Cardiol. 1987;10:1032–9.
- Lindqvist P, Henein M, Kazzam E. Right ventricular outflow-tract fractional shortening: an applicable measure of right ventricular systolic function. Eur J Echocardiography. 2003;4:29–35.
- Hammarstrom E, Wranne B, Pinto FJ, Puryear J, Popp RL. Tricuspid annular motion. J Am Soc Echocardiogr. 1991;4:131–9.
- Rein AJ, Lewis N, Forst L, Gotsman MS, Lewis BS. Echocardiography of inferior vena cava in healthy subjects and in patients with cardiac disease. Isr J Med Sci. 1982;18:581–5.
- Minutiello L. Noninvasive evaluation of central venous pressure derived from respiratory variations in the diameter of the inferior vena cava. Minerva Cardioangiol. 1993;41:433–7.
- Byrd BF, Linden RW. Superior vena cava Doppler flow velocity patterns in pericardial disease. Am J Cardiol. 1990;65:1464–70.
- Bibra HV, Schober K, Jenni R, Busch R, Sebening H, Blomer H. Diagnosis of constrictive pericarditis by pulsed Doppler echocardiography of the hepatic vein. Am J Cardiol. 1989;63:483–8.
- Klein AL, Hatle LK, Burstow DJA, et al. Comprehensive Doppler assessment of right ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990;15:99–108.
- Oh JK, Seward JB, Tajik AJ. Assessment of diastolic function and diastolic heart failure. In: The Echo Manual (3rd edn.). Oh JK, Seward JB, Tajik AJ (ed.). Philadelphia: Lippincott Williams & Wilkins; 2006:120–42.
- Oh JK, Seward JB, Tajik AJ. Pulmonary hypertension and pulmonary vein stenosis. In: The Echo Manual (3rd edn.). Oh JK, Seward JB, Tajik AJ (ed.). Philadelphia: Lippincott Williams & Wilkins; 2006:143–53.
- Miller D, Farah MG, Liner A, Fox K, Schluchter M, Hoit BD. The relation between quantitative right ventricular ejection fraction and indices of tricuspid annular motion and myocardial performance. J Am Soc Echocardiogr. 2004;17:443–7.
- Rushmer R, Crystal D, Wagner C. The functional anatomy of ventricular contraction. Circulation Res. 1953;1:162–70.
- Koestenberger M, Ravekes W, Evertt AD, et al. Right ventricular function in infants, children and adolescents. Reference values of the tricuspid annulus plane systolic excursion (TAPSE) in 640 healthy patients and calculation of Z score values. J Am Soc Echocardiogr. 2009;22:715–9.
- Alp H, Karaqrsln S, Baysal T, Cimen D, Ors R, Oran B. Normal values of left and right ventricular functions measured by M-Mode, pulsed Doppler and Doppler tissue imaging in healthy term neonates during 1-year period. Early Hum Develop .2012;88:853–9.
- Tuller D, Steiner M, Wahl A, Kabok M, Seiler C. Systolic right ventricular function assessment by pulsed wave tissue Doppler imaging of the tricuspid annulus. Swiss Med Wkly. 2005;135:461–8.
- Fahmy Einoamany M, Abdelraouf Dawood A. Right ventricular myocardial isovolumic relaxation time as novel method for evaluation of pulmonary hypertension: correlation with endothelin 1 level. J Am Soc Echocardiogr. 2007;20:462–9.
- Galderisi M, Severino S, Cicala S, Caso P. The usefulness of pulsed tissue Doppler for the clinical assessment of right ventricular function. Ital Heart J. 2002;3:241–7.
- Fan Y, Shen JX, Yang SS, et al. Evaluation of right ventricular function by tissue Doppler echocardiography and Tei index in right ventricular myocardial infarction. Zhonghua Nei Ke Za Zhi. 2005;44:180–3.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528