Atrial Fibrillation: Clinical and Epidemiological Aspects
Volume 3, Apr 2014
Kartikeya Bhargava, MD, DNB, Gurgaon, India
J Clin Prev Caridiol. 2014;3(2):55-60
Introduction
ECG Patterns during AF
- The chaotic atrial activation during AF results in either complete absence of P-waves or more commonly presence of fibrillatory waves (instead of the normal P-waves) that vary in rate and morphology from beat to beat (F-waves). This is associated with irregular and frequently fast ventricular rate. The QRS morphology is usually normal, though widened QRS complexes may be seen if there is underlying bundle branch block or aberrant intraventricular conduction occurs during AF. Another rare but important cause of wide QRS complexes during AF is preexcited AF wherein an atrioventricular accessory pathway is present (Wolff–Parkinson–White [WPW] syndrome) and conducts antegradely during AF. The ventricular rate is very rapid and QRS complexes very broad in the background of markedly irregular RR intervals in preexcited AF. It is important to realize that the ventricular rhythm is usually irregular during AF. A regular slow ventricular rhythm during AF suggests complete AV block, while AF and regular fast ventricular rhythm with wide QRS complexes suggesting ventricular tachycardia along with AF.
Occasionally, and especially after anti-arrhythmic drug therapy, AF may convert to atrial flutter. Also, at times atrial flutter may degenerate into AF and hence ECG pattern in the same patient may fluctuate between AF and atrial flutter. During atrial flutter, the atrial rate varies from 250 to 350 beats per minute and the flutter waves are typically uniform with the absence of any isoelectric interval between two consecutive flutter waves. AF can sometimes be misdiagnosed as atrial flutter (5), especially if lead V1 shows prominent P-waves, though a more careful look indicates changing morphology of the P-waves. Occasionally, atrial tachycardia may also be seen in patients with paroxysmal AF. It is characterized by an atrial rate greater than 100/min with distinct discrete and uniform P-waves. The rate and regularity of ventricular rhythm depends on the degree of atrioventricular conduction that can be 1 to 1 or 2 to 1 or more commonly variable.
Classification of Atrial Fibrillation
Many methods of classification of AF have been described though the one clinically useful is presented here. This is based on the presentation and the duration of the arrhythmic event/s (6).
- First detected AF: A patient who presents with documented AF for the first time is designated as first detected or first diagnosed AF irrespective of the duration of arrhythmia and presence of AF-related symptoms.
- Paroxysmal AF: Self-terminating episodes of AF that last less than 7 days and usually less than 48 hours are denoted as paroxysmal AF.
- Persistent AF: When an episode of AF lasts longer than 7 days or requires cardioversion with drugs or direct current shock for termination, it is designated as persistent AF. Two or more episodes of AF in a patient are called recurrent AF that can be either paroxysmal or persistent. It is to be noted that these designations are in no way mutually exclusive; a patient with usually paroxysmal AF may have an episode of persistent AF and vice versa. The patient should be designated to have paroxysmal or persistent AF based on the predominant presentation.
- Long-standing persistent AF: When an episode of AF has been present for more than a year and a rhythm control strategy is adopted, it is designated as long-standing persistent AF.
- Permanent AF: When it is accepted that AF will persist since cardioversion has failed, or is not desirable or not attempted, the AF is said to be of permanent form. In this form, by definition, rhythm control interventions are not undertaken. If it is decided to terminate AF by drugs or direct current cardioversion, then its designation is changed to long-standing persistent AF.
Certain other terms are commonly used to describe AF and include the following:
Silent AF: Asymptomatic episodes of AF may be detected incidentally in an ECG or due to a complication of AF like stroke. Usually even in patients with symptomatic paroxysmal AF, many episodes of AF are silent and are detected by long-term ECG monitoring. One-third of patients with AF do not have symptoms and hence have silent AF.
Secondary AF: AF occurring due an underlying reversible cause like acute myocardial infarction, cardiac surgery or pneumonia is called secondary AF, since treatment of the underlying cause along with the management of episode of AF usually is enough for restoring sinus rhythm. Long-term management including anticoagulation in this subset of AF in not needed provided the patient does not have episodes of AF at other times independent of the underlying transient disorder.
Lone AF: Occurrence of AF in a young patient (<60 years of age) who does not have any cardiopulmonary disease or hypertension is known as lone AF (7). This subset of AF patients has very low risk of stroke and thromboembolism and hence a favorable long-term prognosis.
Valvular AF: AF occurring in patients with rheumatic heart disease (RHD), mitral valve disease of other etiologies, prosthetic heart valve or mitral valve repair carries high risk of stroke requiring long-term oral anticoagulation and has been classified separately. Most studies of oral anticoagulation and stroke risk in the Western population have been performed in non-valvular AF.
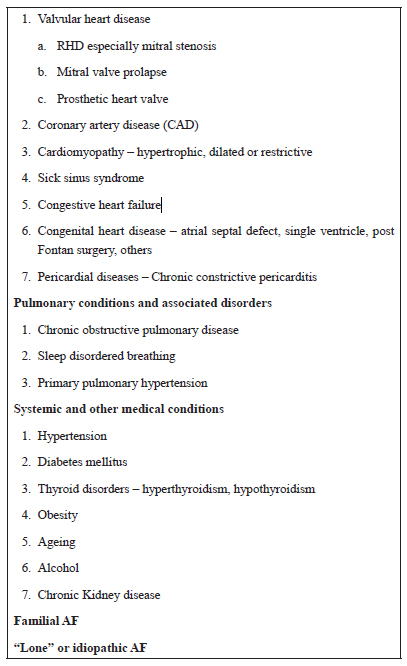
Table 1. Causes and Conditions Associated with Atrial Fibrillation
Epidemiology of Atrial Fibrillation
AF is the most common sustained arrhythmia in general practice with a prevalence of 0.4–1% of the general population (1) that increases strikingly with increasing age. It has always been known that rheumatic valvular heart disease is the most common cause of AF in India and other Asian countries compared to hypertension which is the most common associated disease in western countries.
A small study in rural India found that the RHD was the commonest cause of AF (61.31%), followed by hypertensive heart disease and chronic obstructive pulmonary disease (9).
Many more interesting data on epidemiology of AF emerged from the RE-LY AF registry (8) – a prospective registry of more than 15,000 patients with AF in 46 countries belonging to various income groups. It demonstrated a wide regional variation in the risk factors, concomitant diseases and treatment strategies of AF. Although hypertension was the most common risk factor for AF globally, RHD was a major cause of AF in Africa, China, the Middle East and India where it accounted for almost one-third of patients with AF. RHD was present in 11.6% of patients globally, but only in 1.5% of patients in Western Europe and 2.2% in North America compared to 31.5% in India (8).
In the RE-LY AF registry, the patients with AF in India, the Middle East and Africa were on average 10–12 years younger than the other regions of the world (8).
The type of AF also varied among the regions of the world – paroxysmal, persistent and permanent AF were nearly equally distributed in Western Europe whereas in Africa, the Middle East, China and India, there was large percentage of patients with permanent AF (81.4%, 71.7%, 54.8% and 46.6%, respectively in the four regions). The percentage of AF patients categorized as lone AF was low overall varying from 4.4% to 17.9% in various regions with India having a figure of 6.3% (8).
Overall, a history of stroke or transient ischemic attack (TIA) was present in 13.8% of patients presenting with AF though the rate was much lower (7.4%) in India (8).
The registry also showed that the use of oral anticoagulation in patients with AF was much lesser than desired varying from 13.7% to 43.8% and even lesser in countries like India (23.5%) (8).
AF: Mechanisms and Pathophysiology
The initiation and maintenance of any sustained arrhythmia including AF requires an arrhythmogenic substrate, potential triggers and precipitating or modulating factors.
The structural and electrical abnormalities produced by associated conditions or causative disorders alter the atrial tissue to promote abnormal impulse formation and propagation resulting finally in AF (10). The structural abnormalities include inflammation, myocardial fibrosis (11) and hypertrophy that can occur as a result of anydisease like CAD or valvular heart disease or heart failure that tend to increase atrial pressure, cause atrial dilation and alter wall stress. Even rapid atrial rates as in clinical atrial tachycardia or experimental atrial pacing result in structural alterations in the atrial musculature that heterogeneously alter impulse conduction and refractoriness producing an arrhythmogenic substrate.
The triggers that initiate AF are usually focal ectopic discharges emanating most commonly from the myocardial sleeves in the pulmonary veins (12) but can arise from other atrial or venous locations as well. The unique anatomic and electrophysiologic features of the pulmonary vein musculature make these regions arrhythmogenic in nature (12,13). Reentry, automaticity and triggered activity – any of the three mechanisms of arrhythmogenesis in the pulmonary veins – may promote rapid focal firing that can initiate AF which then becomes sustained in the presence of appropriate atrial substrate and the biochemical milieu. Although the pulmonary veins are the most common sites for triggers, other triggering sites can include coronary sinus, vena cava, appendages and posterior left atrium.
The exact mechanisms that are responsible for maintenance of AF are not clearly understood but may include (a) multiple reentrant wavelets, (b) multiple rapidly firing foci, or (c) multiple rotors or spiral wave reentrant circuits (13). Even multiple mechanisms may be operative in an individual with AF.
Newer insights in the mechanism of AF have implicated an important role of the autonomic nervous system (ANS) (14). In fact, some believe that heart has its own nervous system and the presence of ganglionic plexi in the atria that are considered a part of ANS have been target of ablation for AF in some studies (15). Also, in some patients AF gets provoked during conditions of high parasympathetic tone like sleep and is known as vagally mediated AF. Similarly, high sympathetic activity such as exercise may precipitate AF in certain other patients (16).
Pathophysiologic Mechanisms
Many different underlying pathophysiological mechanisms may be operative singly or in combination in patients with AF. These include atrial structural remodeling (e.g., AT-induced AF, AF begets AF), inflammation and oxidative stress (as in cardiacsurgery, pericarditis, aging), and activation of rennin-angiotensin system (as in hypertension). In addition, many clinical (age, hypertension, diabetes, heart failure, etc.), electrocardiographic (left atrial abnormality, left ventricular hypertrophy), echocardiographic (left atrial enlargement, increased left ventricular wall thickness) and biochemical (brain natriuretic peptide, high-sensitivity C Reactive Protein) markers are associated with an increased risk of AF (10).Reversible AF
Cure of AF even if recurrent by elimination or treatment of underlying cause has supported the notion of reversible AF. These potentially reversible causes of AF include acute myocardial infarction, thyrotoxicosis, cardiac and noncardiac surgery, binge alcohol intake, pulmonary embolism and WPW syndrome. These patients may not need long-term treatment including anticoagulation for AF after elimination of the cause, although clinical followup is advisable to ascertain recurrence of AF.
AF: Clinical Features, Presentation and Evaluation
AF may at times be asymptomatic or may present with varying symptoms that can be grouped as shown next. In a given patient with symptomatic AF, there may be many more episodes of AF that are asymptomatic (17). Absent or unreliable symptoms are more common among the elderly, whereas the young patients usually recall the symptoms of palpitations due to rapid and irregular beating of the heart.
A. Symptoms due to AF per se
- Palpitations. AF results in irregular and often very rapid beating of the heart that produces the symptoms of palpitations. The patients, especially those who are young, may remember and may categorically define the irregular nature of their heart beats.
- Breathlessness. Rapid ventricular rate during AF leads to shortening of the diastolic filling time in the ventricles with resultant reduction in cardiac output that may present as dyspnea. Also, loss of effective atrial contraction eliminates the atrial contribution to the cardiac output (usually 20%) especially in patients with stiff ventricles like in elderly or in those with hypertension. This may result in breathlessness and even heart failure. Breathlessness may also result from left ventricular systolic dysfunction due to tachycardiomyopathy as described next.
- Angina. AF in patients with CAD may cause angina by reducing the coronary blood flow due to shortened diastole and precipitating ischemia. Moreover, studies have shown that coronary blood flow is reduced during irregular ventricular rhythm in AF compared with a regular rhythm at the same average rate (18) possibly related to the sympathetically mediated coronary vasoconstriction.
- Fatigue, lightheadedness or syncope. These symptoms are more common when AF occurs in patients with sinus node dysfunction. Syncope can occur due to a pause that occurs at termination of AF in some patients.
B. Symptoms due to complications of AF
- Stroke. Ineffective atrial contraction during AF results in stasis of blood in the atria that can lead to formation of thrombus especially in the left atrial appendage. Embolization of thrombus to intracranial circulation can result in embolic stroke with resultant loss of motor, sensory or other function. The stroke associated with AF is more often larger, more disabling and more likely to be fatal. Hence, stroke is the most important complication of AF and its risk can be effectively reduced by long-term anticoagulation therapy. It is important to recognize that the risk of stroke is similar whether the AF is paroxysmal, persistent or permanent and is largely determined by associated comorbid conditions.
- Thromboembolism. Emoblization of atrial thrombus to systemic circulation other than intracranial can result in symptoms and signs related to the region of compromised circulation like peripheral limb ischemia, mesenteric ischemia or splenic infarction.
- Tachycardiomyopathy. Rapid and irregular ventricular rates during AF can result in decline in left ventricular systolic function that gets reversed completely or partially with control in rate or rhythm – a condition known as tachycardiomyopathy (19). The potential for reversibility is inversely related to the duration of rapid heart rate; longer lasting AF with rapid ventricular rates is likely to have only partial incomplete recovery following normalization of heart rates.
- Heart failure. The patients with preexisting heart disease or left ventricular dysfunction may present with worsening of heart failure with onset of AF due to all the mechanisms described earlier. The patients with diastolic dysfunction due to conditions like hypertrophic cardiomyopathy often do not tolerate AF and rapidly worsen.
C. Symptoms and signs due to underlying cardiac or systemic diseasem
Finally, a patient with AF may present due to symptoms or signs of underlying disease like CAD or thyrotoxicosis or pulmonary disease. The symptoms of AF per se are absent and clinical evaluation and investigations for the other disease bring out the incidental diagnosis of AF as well.
Evaluation of AF
The diagnosis of AF in a patient is based on clinical history and examination and is confirmed by electrocardiographic diagnosis made possible by ECG, Holter monitor, loop recorder or interrogation of pacemaker. The evaluation of a patient diagnosed with AF is based on the following principles:
- Diagnosis of AF and differential diagnosis with other arrhythmias
- Characterizing the pattern of AF – paroxysmal, persistent or permanent
- Detection of any reversible causes
- Determination of underlying cause or associated cardiac or systemic disease
- Assessment of thromboembolic risk and bleeding risk
- Investigations to guide therapy including transesophageal echocardiography to detect atrial thrombi
Conclusions
AF is the most common sustained arrhythmia in the clinical practice and assumes high importance due to the associated risk of stroke and thromboembolism. There is a marked heterogeneity and regional variation in the underlying causes, clinical presentation and treatment patterns in AF. Since it may be asymptomatic, its detection even in absence of symptoms by clinical evaluation and appropriate investigations is important to guide appropriate therapy in order to reduce the risk of stroke.
Conflicts of Interest
None
Acknowledgments
None
References
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention. The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–5.
- Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–73.
- Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–57.
- Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–64.
- Knight BP, Michaud GF, Strickberger SA, Morady F. Electrocardiographic differentiation of atrial flutter from atrial fibrillation by physicians. J Electrocardiol. 1999;32:315–9.
- ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2006;48:e149—246.
- Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR Jr, Ilstrup DM, Frye RL. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669–74.
- Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P, Zhu J, Jansky P, Sigamani A, Morillo CA, Liu L, Damasceno A, Grinvalds A, Nakamya J, Reilly PA, Keltai K, Van Gelder IC, Yusufali AH, Watanabe E, Wallentin L, Connolly SJ, Yusuf S; RE-LY Atrial Fibrillation Registry Investigators. Variations in cause and management of atrial fibrillation in a prospective registry of 15400 emergency department patients in 46 countries. The RE-LY atrial fibrillation registry. Circulation. 2014;129:1568–76.
- Bhardwaj R. Atrial fibrillation in a tertiary care institute – A prospective study. Indian Heart J. 2012;64:476–8.
- January CT, Wann SL, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014; (in press)
- Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–9.
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66.
- Jais P, Hocini M, Macle L, Choi KJ, Deisenhofer I, Weerasooriya R, Shah DC, Garrigue S, Raybaud F, Scavee C, Le Metayer P, Clémenty J, Haïssaguerre M. Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation. 2002;106:2479–85.
- He B, Scherlag BJ, Nakagawa H, Lazzara R, Po SS. The intrinsic autonomic nervous system in atrial fibrillation: A review. ISRN Cardiol. 2012;2012:490674.
- Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–34.
- Patton KK, Zacks ES, Chang JY, Shea MA, Ruskin JN, Macrae CA, Ellinor PT. Clinical subtypes of lone atrial fibrillation. Pacing Clin Electrophysiol. 2005;28:630–8.
- Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224–7.
- Van Den Berg MP, Tuinenburg AE, van Veldhuisen DJ, de Kam PJ, Crijns HJ. Cardioversion of atrial fibrillation in the setting of mild to moderate heart failure. Int J Cardiol. 1998;63:63–70.
- Grogan M, Smith HC, Gersh BJ, Wood DL. Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;69:1570–3.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528


