The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Review Article
The Use of Three-dimensional Echo in Clinical Practice
Volume 1, Jan 2012
Carly Jenkins, Sudhir Wahi; Queensland, Australia
Page: 11-9.
The rationale for the use of three-dimensional echocardiography (3DE) in a clinical setting is growing. The four main areas that have investigated the value of 3DE include the analysis of cardiac volumes and left ventricular (LV) mass, ischemic heart disease, congenital heart disease, and valvular pathology. Although various versions of 3DE have now been in use since the early 1970s (1), “live” 3DE has only been in use since the early 2000s.3DE can display, in real time, the views and motions of deeper cardiac structures, which are unavailable by two-dimensional echocardiography (2DE) and is therefore capable of providing superior diagnostic information. This is particularly advantageous given the complex spatial relations of cardiac structures especially in the fields of acquired valvular disease (2), atrial and ventricular septal defects (3), and LV remodeling (4). It can also display exact assessments of left atrial volume (5,6), LV volumes and function, and volumetric measurements of irregularly shaped geometric chambers such as right ventricle. The ability of 3DE to simulate surgical views helps to facilitate vital surgical decisions such as the accurate assessments of the effect of percutaneous balloon valvuloplasty and the function of prosthetic valves and septal occluders. Finally it may help in making more accurate qualitative diagnosis and classification of congenital heart disease (7).
Both qualitative and quantitative limitations of the two-dimensional (2D) imaging have led to the emergence of the 3DE technique. Until recently, the 3DE technology has been slow due to image quality and longer processing and acquisition times compared with 2DE. The most recent of the 3DE technologies is the “live” or “real-time” three-dimensional (RT3D) technique; however, there have been a number of systems and methods that led up to this recent advancement.
Evolution of Three-Dimensional Echocardiography
The first step in the development of “real-time” 3DE was the development of external tracking or freehand scanning. In 1973, Dekker et al. (8) were the first to demonstrate hardware capable of collecting three-dimensional (3D) cardiac ultrasound. This consisted of four-dimensional data collection and display subsystems. It used a large mechanical arm that measured the probe displacement during 2D image acquisitions. Even though this was a new and exciting technique and even a nonskilled worker could use the machine, it was time-consuming and very impractical (8). The next group to expand on the 3D tracking method was Brinkley et al. in 1978 (9). This group developed an acoustic locator or “spark gap” where regular audio pulses are sent attached to the ultrasound probe and detected by a fixed antenna.
Following this, a “freehand” scanning device was developed which was attached to the ultrasound probe allowing continuous scanning of the heart in space. Freehand scanning was developed in 1979 and is still used in some clinical laboratories today. This device uses a magnetic field system, which orients sequentially acquired 2DE images by tracking the movement of the ultrasound probe used by the sonographer. However, this system is time-consuming and relatively immobile because of the associated receiving computer where the image is reconstructed. Gated sequential imaging was another method for 3DE. This modality worked under the assumption that both the patient and the ultrasound probe remain in a fixed position. Using three transducer methods (rotational, fan-like, and linear scanning), aligned cuts of the heart are obtained and reconstructed (10). This method, however, fundamentally relies on maintaining adequate 2DE image quality over a long acquisition time and, therefore, is subject to heart rate variability, lung artifact, and respiratory rate.
In the early 1990s, the “real-time” volumetric 3DE was developed by Duke University using a sparse matrix array transducer. This technique is based on the concept that the heart would fit into a pyramidal dataset and does not rely on the transducer movement or sequential capturing. Although the output is known as “real-time,” the output actually consists of multiple 2D images displayed simultaneously (11).
This technique formed the basis of the newest three-dimensional technique – “real-time” or “live” 3DE. RT3D uses a 3.5 MHz transducer with 256 firing elements in the form of a 2D grid. The elements are arranged in a grid instead of a line that enables acquisition of an on-line three-dimensional volume of ultrasound data.
The previous three-dimensional transducers used a modified two-dimensional probe that had elements arranged in a single line. The advantage with RT3D is that the transducer does not move to obtain data, unlike previous models where multiple windows and planes were needed to reconstruct an image.
RT3D uses linked images from four cardiac cycles gated from an electrocardiograph (ECG). However, image quality remains limited because of the number of transducer elements, transducer frequency, image depth, and processing power. This leads to lower spatial and temporal resolution compared with other techniques. The matrix array transducer includes up to 3000 individual elements and a faster processing speed, which also allows for “live” 3D imaging. This is a useful tool, not only for three-dimensional viewing but also for biplane 2DE where two simultaneous perpendicular images can be viewed side by side.
The development of the 3D transesphageal imaging probe is similar to the trans-thoracic probe, allowing imaging in three different modes. Firstly, there is a narrow sector acquisition, which is a 50o × 30o pyramid. Secondly, there is a 3D zoom, which shows a smaller but magnified 85o × 85o pyramid. Lastly the full pyramid sector which is similar to the trans-thoracic echo (TTE) probe in which four consecutive beats make up a 90o × 90o pyramid
Assessment of Left Ventricular Function
The quantification of LV volumes and ejection fraction (EF) is an important aspect of cardiac evaluation in all cardiac disorders. Indeed assessment of LV function is perhaps the most common of all indications to request an echocardiogram. The serial assessment of LV function is frequently used to guide therapy. However, repeated measurements are prone to variation due to poor image quality, geometric issues related to volume and mass calculations, the performance of measurements from off-axis cuts, and variations in ventricular loading (12). EF is a simple numerical value that reflects LV function. However, trans-thoracic 2DE has limited test–retest reliability (13). Studies have shown that Simpson’s biplane calculation of EF can vary up to 4.1% between readers (14,15). This variability is due to the complex geometric assumptions and potential problems with image foreshortening that 2DE calculation of EF encounters.
Consequently, cardiac magnetic resonance imaging (MRI) has been proposed as a more desirable alternative for LV assessment, especially in clinical trials (16) because of its good image quality and high spatial resolution. Given this, cardiac MRI has become the “gold standard” for LV volumes, EF, and LV mass. However, expense, patient intolerance (e.g., claustrophobia, noise), a relative contraindication in patients with cardiac devices, and lack of portability have limited the use of this modality in routine clinical practice.
To overcome many of the geometric assumptions and to counteract the issues related to LV axial alignment, in the last three decades, 3DE has developed into clinical tool for measuring volumes, EF, and LV mass. Many studies have shown that 3DE is more closely correlated to MRI with less variability than 2DE for cardiac measurements (Fig. 1). The advancement of 3DE from a cumbersome offline tool to a “real-time” online process has taken it out of the research arena and into the clinical laboratory.
3DE has the advantage of accurate delineation of the true long axis length of the ventricle, thereby increasing the accuracy of Simpson’s guided biplane measurements. For 2DE, the accuracy of LV volumes by Simpson’s method is dependent on the apical four- and two-chamber lengths being nearly equal. Since the geometric assumptions of the 2DE calculations depend on the accuracy of ventricular lengths, foreshortening will result in underestimation of the cross-sectional area and thereby volumes. Recent advancements in 3DE technology have allowed for faster assessment in full LV volume measurements due to the semi-automated endocardial edge detection. Online measurement of 3D LV volumes is feasible and more accurate than with 2DE (17).
.jpg)
3DE has the advantage of accurate delineation of the true long axis length of the ventricle, thereby increasing the accuracy of Simpson’s guided biplane measurements. For 2DE, the accuracy of LV volumes by Simpson’s method is dependent on the apical four- and two-chamber lengths being nearly equal. Since the geometric assumptions of the 2DE calculations depend on the accuracy of ventricular lengths, foreshortening will result in underestimation of the cross-sectional area and thereby volumes. Recent advancements in 3DE technology have allowed for faster assessment in full LV volume measurements due to the semi-automated endocardial edge detection. Online measurement of 3D LV volumes is feasible and more accurate than with 2DE (17).
.jpg)
The calculation of LV volumes and function underpin important clinical decisions in patient management. This increasing application and monitoring of devices in heart failure, for example implantable defibrillators (AICD), cardiac resynchronization therapy (CRT), LV remodeling surgery, and in future stem cell therapy, require more accurate assessment of LV remodeling. The current American College of Cardiology and American Heart Association guidelines for the management of heart failure recommend the use of LV dimensions and 2DE EF for this purpose (18). A number of studies have shown that 3DE has overcome many of the limitations of 2DE with less test–retest variation, better reproducibility, and accuracy in LV volume estimations. A recent study by Hare et al. (19) has shown differences in the classification of patients into EF thresholds with 3DE compared to 2DE, which may impact treatment decisions, especially regarding device therapy. Moreover, 3DE appears to be superior to 2DE for evaluating LV size in long-term follow-up. Recently, there has been an attempt to validate a standardized 3DE protocol for measuring LV volumes and EF. The first multicenter study to validate and provide information of the sources of error between MRI and 3DE found that the major source of error of 3DE is the definition of endocardial borders. In 3DE, the trabeculae are blended in with the myocardium rather than being included in the LV cavity, as is the case with MRI. Another critical difference is that MRI uses short-axis slices whereas 3DE uses long-axis slices and both use separate software for analysis. This was investigated with the use of a phantom which found very small differences between the techniques for measured volumes (20). 3DE is now the gold standard echocardiographic measurement of choice for the accurate calculation of LV volumes and EF. As demand for more reproducible measurements is sought to guide management decisions, the demand for 3DE will grow accordingly and is likely to be soon incorporated into mainstream cardiac guidelines.
.jpg)
Quantification of global LV function is important; however, in patients with heart failure there is potential for LV dyssynchrony and regional analysis. Previous techniques for assessment of intra-ventricular dyssynchrony including tissue Doppler and M-mode analysis have been used to assess the LV on a per segment basis. These methods used to evaluate LV mechanical dyssynchrony are technically difficult and do not assess the whole LV simultaneously. RT3D can quantify global mechanical dyssynchrony. LV mechanical synchrony has emerged as a therapeutic target using cardiac resynchronization therapy in selected patients with chronic heart failure. RT3D represents a new technique to identify chronic heart failure patients, previously not considered suitable for resynchronization therapy, who might benefit from such therapy (Fig. 2). The first of the dyssynchrony studies using 3D was published in 2005 and found a systolic dyssynchrony index for both normal EF and abnormal EF populations (4). It has also been found to have high sensitivity and specificity (21) and good reproducibility at centers (22).
Assessment of Valvular and Congenital Abnormalities
Three-dimensional echocardiography and in particular 3D trans-esophageal
echocardiography (3D-TEE) has become a critical diagnostic tool for patients with valvular abnormalities. In mitral valve assessment, 3D-TEE has allowed visual analysis of the leaflets scallops, chordate, and shape of the annulus (23,24). It allows easy visualization of the valve from a surgical view and accurate diagnosis of pathology such as stenosis (25), prolapsed segments (23) (Fig. 3), and regurgitation (26).
.jpg)
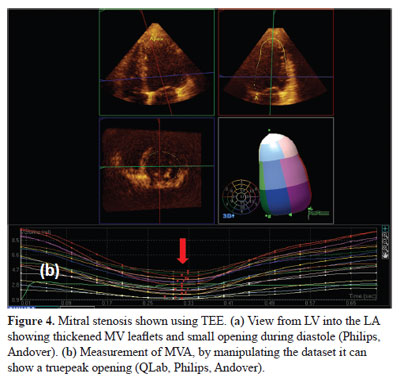
In patients with mitral stenosis, a 3DE dataset can be manipulated to show the true orifice area (Fig. 4). Studies have shown that 3DE has a better correlation than 2DE to invasive methods when assessing mitral valve areas (2,25). 3DE has also shown to play an important role in pre- and post-percutaneous balloon mitral valvuloplasty (27).
.jpg)
3DE allows superior visualization of all mitral valve apparatus and leaflets scallops than 2DE and is particularly useful in conditions such as Barlow’s disease (28). 3DE can accurately show each prolapsing segments and aid in the decision to proceed to either mitral valve repair or replacement. In mitral regurgitation cases, 3DE has been shown to provide information of jet origin and orientation, improve calculation of flow convergence areas, and effective regurgitant orifice areas (26,29). In functional mitral regurgitation, the vena contracta width and area can vary significantly depending on image orientation. 3DE derived vena contracta area has been shown to correlate better with severity of mitral regurgitation, and well emerge as a new quantification tool (26). Paravalvular leaks are a post-surgical complication of valvular replacement or repair. 3D-TEE is superior to 2D-TEE in determining the number, locations, and shape of the leak (30) (Fig. 5). 3D-TEE is now the method of choice for guiding repair of paravalvular leaks, in positioning prosthetic devices into the defect, and assessing any residual leaks or interference to the valve leaflets (31).
(4).jpg)
(1).jpg)
(1).jpg)
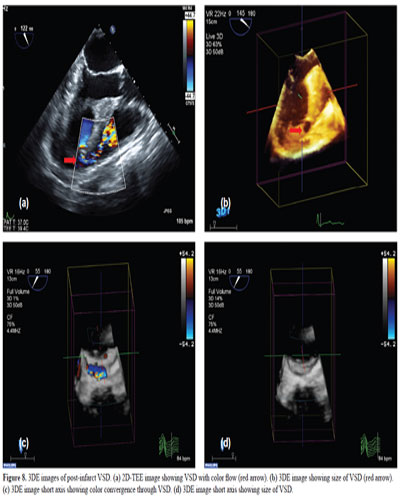
In patients with congenital heart disease (especially atrial septal, or ventricular septal defects, ASD or VSD), 3D can show the location, size, configuration, type, and motion of the defect (Figs. 6–8). 3D also shows the spatial relations of the defect with the neighboring structures and the image can be rotated to view the defect from either the left or the right side of the septum. Studies have shown that 3D is a technology that allows instant visualization of cardiac anatomic details that could not be well-delineated by 2D imaging (32). It is also now feasible to guide percutaneous ASD and VSD closures with the use of 3D-TEE (33–35). 3D-TEE has the advantage over 2D by accurately measuring rim lengths, distance between multiple defects, and localization of the defect in relation to other structures such as the aortic or mitral valvular apparatus, aortic root, pulmonary veins, and the vena cava. Not only has TEE been shown to aid in the evaluation of complex congenital defects (36) but also in detection of intracardiac masses, tumors (37) and thrombus, and the evaluation of structure such as the left atrial appendage (LAA) (38). 3D-TEE has become the imaging modality of choice to size the device for LAA occlusion and to guide the procedure safely (39).
(4).jpg)
(1).jpg)
.jpg)
(3).jpg)
(2).jpg)
Key Uses of Three Dimensional Echocardiography
Conflict of Interest
.jpg)
In patients with mitral stenosis, a 3DE dataset can be manipulated to show the true orifice area (Fig. 4). Studies have shown that 3DE has a better correlation than 2DE to invasive methods when assessing mitral valve areas (2,25). 3DE has also shown to play an important role in pre- and post-percutaneous balloon mitral valvuloplasty (27).
.jpg)

3DE allows superior visualization of all mitral valve apparatus and leaflets scallops than 2DE and is particularly useful in conditions such as Barlow’s disease (28). 3DE can accurately show each prolapsing segments and aid in the decision to proceed to either mitral valve repair or replacement. In mitral regurgitation cases, 3DE has been shown to provide information of jet origin and orientation, improve calculation of flow convergence areas, and effective regurgitant orifice areas (26,29). In functional mitral regurgitation, the vena contracta width and area can vary significantly depending on image orientation. 3DE derived vena contracta area has been shown to correlate better with severity of mitral regurgitation, and well emerge as a new quantification tool (26). Paravalvular leaks are a post-surgical complication of valvular replacement or repair. 3D-TEE is superior to 2D-TEE in determining the number, locations, and shape of the leak (30) (Fig. 5). 3D-TEE is now the method of choice for guiding repair of paravalvular leaks, in positioning prosthetic devices into the defect, and assessing any residual leaks or interference to the valve leaflets (31).
(4).jpg)
(1).jpg)
(1).jpg)
In patients with congenital heart disease (especially atrial septal, or ventricular septal defects, ASD or VSD), 3D can show the location, size, configuration, type, and motion of the defect (Figs. 6–8). 3D also shows the spatial relations of the defect with the neighboring structures and the image can be rotated to view the defect from either the left or the right side of the septum. Studies have shown that 3D is a technology that allows instant visualization of cardiac anatomic details that could not be well-delineated by 2D imaging (32). It is also now feasible to guide percutaneous ASD and VSD closures with the use of 3D-TEE (33–35). 3D-TEE has the advantage over 2D by accurately measuring rim lengths, distance between multiple defects, and localization of the defect in relation to other structures such as the aortic or mitral valvular apparatus, aortic root, pulmonary veins, and the vena cava. Not only has TEE been shown to aid in the evaluation of complex congenital defects (36) but also in detection of intracardiac masses, tumors (37) and thrombus, and the evaluation of structure such as the left atrial appendage (LAA) (38). 3D-TEE has become the imaging modality of choice to size the device for LAA occlusion and to guide the procedure safely (39).
(4).jpg)
(1).jpg)
.jpg)
(3).jpg)
(2).jpg)

Key Uses of Three Dimensional Echocardiography
1. Volume and EF analysis
a. For use in serial follow-up on patients who need intracardiac devices, valvular abnormalities, and chemotherapy.
2. Dyssynchrony and regional analysis
2. Dyssynchrony and regional analysis
a. For patients with heart failure and the need for resynchronization therapy
3. Valvular abnormalities
3. Valvular abnormalities
a. Assessment of leaflets scallops, chordae, and shape of the annulus.
b. Surgical view of pathology such as origin of regurgitant jets, prolapsing segments and calculation of valve areas.
4. Congenital abnormities
4. Congenital abnormities
a. Septal defect evaluation and percutaneous device repairs.
b. Detection of intracardiac masses, tumors, and thrombus.
Conclusions
Although 3DE is emerging as the echocardiographic method of choice for LV volume, valvular and congenital abnormality assessment, however, practice guidelines do not yet take cognizance of this evidence. The uptake of this technique into the clinical laboratory has been slow and may be limited by inexperience. An interactive teaching course with rehearsal and direct mentoring appears to overcome this limitation and may improve the uptake of this technique (40).
Both 3D-TEE and TTE are no longer just a research tool but a feasible clinical tool for the diagnosis and measurement of volumes, valvular abnormalities, and structural defects.
Both 3D-TEE and TTE are no longer just a research tool but a feasible clinical tool for the diagnosis and measurement of volumes, valvular abnormalities, and structural defects.
Funding
No external source of funding.
Conflict of Interest
None
References
1. Dekker DL, Piziali RL, Dong E, Jr. A system for ultrasonically imaging the human heart in three dimensions. Comput Biomed Res. 1974; 7:544–53.
2. Zamorano J, Cordeiro P, Sugeng L, Perez de Isla L, Weinert L, Macaya C, Rodríguez E, Lang RM. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: an accurate and novel approach. J Am Coll Cardiol. 2004; 43:2091–6.
3. van den Bosch AE, Ten Harkel DJ, McGhie JS, Roos-Hesselink JW, Simoons ML, Bogers AJ, Meijboom FJ. Characterization of atrial septal defect assessed by real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 2006; 19:815–21.
4. Kapetanakis S, Kearney MT, Siva A, Gall N, Cooklin M, Monaghan MJ. Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation. 2005; 112:992–1000.
5. Artang R, Migrino RQ, Harmann L, Bowers M, Woods TD. Left atrial volume measurement with automated border detection by 3-dimensional echocardiography: comparison with magnetic resonance imaging. Cardiovasc Ultrasound. 2009; 7:16.
6. Jenkins C, Bricknell K, Marwick TH. Use of real-time three-dimensional echocardiography to measure left atrial volume: comparison with other echocardiographic techniques. J Am Soc Echocardiogr. 2005; 18:991–7.
7. Seliem MA, Fedec A, Cohen MS, Ewing S, Farrell PE Jr, Rychik J, Schultz AH, Gaynor JW, Spray TL. Real-time 3-dimensional echocardiographic imaging of congenital heart disease using matrix-array technology: freehand real-time scanning adds instant morphologic details not well delineated by conventional 2-dimensional imaging. J Am Soc Echocardiogr. 2006; 19:121–9.
8. Dekker DL, Piziali RL, Dong E, Jr. A system for ultrasonically imaging the human heart in three dimensions. Comput Biomed Res. 1974;7:544–53.
9. Brinkley JF, Moritz WE, Baker DW. Ultrasonic three-dimensional imaging and volume from a series of arbitrary sector scans. Ultrasound Med Biol. 1978; 4:317–27.
10. Salustri A, Roelandt JR. Ultrasonic three-dimensional reconstruction of the heart. Ultrasound Med Biol. 1995; 21:281–93.
11. von Ramm OT, Smith SW. Real time volumetric ultrasound imaging system. J Digit Imaging. 1990; 3:261–6.
12. Mondelli JA, Di Luzio S, Nagaraj A, Kane BJ, Smulevitz B, Nagaraj AV, Greene R, McPherson DD, Rigolin VH. The validation of volumetric real-time 3-dimensional echocardiography for the determination of left ventricular function. J Am Soc Echocardiogr. 2001; 14:994–1000.
13. Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol. 2004; 44:878–86.
14. Otterstad JE, Froeland G, St John SM, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997; 18:507–13.
15. Otterstad JE. Measuring left ventricular volume and ejection fraction with the biplane Simpson's method. Heart. 2002; 88:559–60.
16. Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995; 8:221–8.
17. Jenkins C, Chan J, Hanekom L, Marwick TH. Accuracy and feasibility of online 3-dimensional echocardiography for measurement of left ventricular parameters. J Am Soc Echocardiogr. 2006; 19:1119–28.
18. Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO. ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr. 2003; 16:1091–110.
19. Hare JL, Jenkins C, Nakatani S, Ogawa A, Yu CM, Marwick TH. Feasibility and clinical decision-making with 3D echocardiography in routine practice. Heart. 2008; 94:440–5.
20. Mor-Avi V, Jenkins C, Kuhl HP, Nesser HJ, Marwick T, Franke A, Ebner C, Freed BH, Steringer-Mascherbauer R, Pollard H, Weinert L, Niel J, Sugeng L, Lang RM. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovasc Imaging. 2008; 1:413–23.
21. Marsan NA, Bleeker GB, Ypenburg C, Van Bommel RJ, Ghio S, Van de Veire NR, Delgado V, Holman ER, van der Wall EE, Schalij MJ, Bax JJ. Real-time three-dimensional echocardiography as a novel approach to assess left ventricular and left atrium reverse remodeling and to predict response to cardiac resynchronization therapy. Heart Rhythm. 2008; 5:1257–64.
22. Kapetanakis S, Bhan A, Murgatroyd F, Kearney MT, Gall N, Zhang Q, Yu CM, Monaghan MJ. Real-time 3D echo in patient selection for cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2011; 4:16–26.
23. Chen X, Sun D, Yang J, Feng W, Gu T, Zhang Z, Xiu Z, Tang L, Ma C, Wang X, Cheng Y, Li N, Liu S. Preoperative assessment of mitral valve prolapse and chordae rupture using real time three-dimensional transesophageal echocardiography. Echocardiography. 2011; 28:1003–10.
24. Pepi M, Tamborini G, Maltagliati A, Galli CA, Sisillo E, Salvi L, Naliato M, Porqueddu M, Parolari A, Zanobini M, Alamanni F. Head-to-head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J Am Coll Cardiol. 2006; 48:2524–30.
25. Schlosshan D, Aggarwal G, Mathur G, Allan R, Cranney G. Real-time 3D transesophageal echocardiography for the evaluation of rheumatic mitral stenosis. JACC Cardiovasc Imaging. 2011; 4:580–8.
26. Kahlert P, Plicht B, Schenk IM, Janosi RA, Erbel R, Buck T. Direct assessment of size and shape of noncircular vena contracta area in functional versus organic mitral regurgitation using real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2008; 21:912–21.
27. Eng MH, Salcedo EE, Quaife RA, Carroll JD. Implementation of real time three-dimensional transesophageal echocardiography in percutaneous mitral balloon valvuloplasty and structural heart disease interventions. Echocardiography. 2009; 26:958–66.
28. Maffessanti F, Marsan NA, Tamborini G, Sugeng L, Caiani EG, Gripari P, Alamanni F, Jeevanandam V, Lang RM, Pepi M. Quantitative analysis of mitral valve apparatus in mitral valve prolapse before and after annuloplasty: a three-dimensional intraoperative transesophageal study. J Am Soc Echocardiogr. 2011; 24:405–13.
29. Fattouch K, Castrovinci S, Murana G, Novo G, Caccamo G, Bertolino EC, Sampognaro R, Novo S, Ruvolo G, Lancellotti P. Multiplane two-dimensional versus real time three-dimensional transesophageal echocardiography in ischemic mitral regurgitation. Echocardiography. 2011; 28:1125–32.
30. Kronzon I, Sugeng L, Perk G, Hirsh D, Weinert L, Garcia Fernandez MA, Lang RM. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. J Am Coll Cardiol. 2009; 53:1543–7.
31. Becerra JM, Almeria C, de Isla LP, Zamorano J. Usefulness of 3D transoesophageal echocardiography for guiding wires and closure devices in mitral perivalvular leaks. Eur J Echocardiogr. 2009; 10:979–81.
32. Seliem MA, Fedec A, Cohen MS, Ewing S, Farrell PE Jr, Rychik J, Schultz AH, Gaynor JW, Spray TL. Real-time 3-dimensional echocardiographic imaging of congenital heart disease using matrix-array technology: freehand real-time scanning adds instant morphologic details not well delineated by conventional 2-dimensional imaging. J Am Soc Echocardiogr. 2006; 19:121–9.
33. Taniguchi M, Akagi T, Watanabe N, Okamoto Y, Nakagawa K, Kijima Y, Toh N, Ohtsuki S, Kusano K, Sano S. Application of real-time three-dimensional transesophageal echocardiography using a matrix array probe for transcatheter closure of atrial septal defect. J Am Soc Echocardiogr. 2009; 22:1114–20.
34. Kijima Y, Taniguchi M, Akagi T, Nakagawa K, Kusano K, Ito H, Sano S. Torn atrial septum during transcatheter closure of atrial septal defect visualized by real-time three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr. 2010; 23:1222–8.
35. Balzer J, Kuhl H, Rassaf T, Hoffmann R, Schauerte P, Kelm M, Franke A. Real-time transesophageal three-dimensional echocardiography for guidance of percutaneous cardiac interventions: first experience. Clin Res Cardiol. 2008; 97:565–74.
36. Zhang YL, Wang M, Xue XP, Hu B. The value of live three-dimensional echocardiography in an adult patient with aortico-left ventricular tunnel. Echocardiography. 2011. Oct 4. [Epub ahead of print]
37. Khairnar P, Hsiung MC, Mishra S, Nanda NC, Daly DD Jr, Nayyar G, Patel A, Mishra J, Chuang YC, Tsai SK, Yin WH, Wei J. The ability of live three-dimensional transesophageal echocardiography to evaluate the attachment site of intracardiac tumors. Echocardiography. 2011; 28:1041–5.
38. Perk G, Biner S, Kronzon I, Saric M, Chinitz L, Thompson K, Shiota T, Hussani A, Lang R, Siegel R, Kar S. Catheter-based left atrial appendage occlusion procedure: role of echocardiography. Eur J Echocardiogr. 2011. Sep 8. [Epub ahead of print]
39. Landmesser U, Holmes DR, Jr. Left atrial appendage closure: a percutaneous transcatheter approach for stroke prevention in atrial fibrillation. Eur Heart J. 2011. Oct 31. [Epub ahead of print]
40. Jenkins C, Monaghan M, Shirali G, Guraraja R, Marwick TH. An intensive interactive course for 3D echocardiography: is 'crop till you drop' an effective learning strategy? Eur J Echocardiogr. 2008; 9:373–80.
2. Zamorano J, Cordeiro P, Sugeng L, Perez de Isla L, Weinert L, Macaya C, Rodríguez E, Lang RM. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: an accurate and novel approach. J Am Coll Cardiol. 2004; 43:2091–6.
3. van den Bosch AE, Ten Harkel DJ, McGhie JS, Roos-Hesselink JW, Simoons ML, Bogers AJ, Meijboom FJ. Characterization of atrial septal defect assessed by real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 2006; 19:815–21.
4. Kapetanakis S, Kearney MT, Siva A, Gall N, Cooklin M, Monaghan MJ. Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation. 2005; 112:992–1000.
5. Artang R, Migrino RQ, Harmann L, Bowers M, Woods TD. Left atrial volume measurement with automated border detection by 3-dimensional echocardiography: comparison with magnetic resonance imaging. Cardiovasc Ultrasound. 2009; 7:16.
6. Jenkins C, Bricknell K, Marwick TH. Use of real-time three-dimensional echocardiography to measure left atrial volume: comparison with other echocardiographic techniques. J Am Soc Echocardiogr. 2005; 18:991–7.
7. Seliem MA, Fedec A, Cohen MS, Ewing S, Farrell PE Jr, Rychik J, Schultz AH, Gaynor JW, Spray TL. Real-time 3-dimensional echocardiographic imaging of congenital heart disease using matrix-array technology: freehand real-time scanning adds instant morphologic details not well delineated by conventional 2-dimensional imaging. J Am Soc Echocardiogr. 2006; 19:121–9.
8. Dekker DL, Piziali RL, Dong E, Jr. A system for ultrasonically imaging the human heart in three dimensions. Comput Biomed Res. 1974;7:544–53.
9. Brinkley JF, Moritz WE, Baker DW. Ultrasonic three-dimensional imaging and volume from a series of arbitrary sector scans. Ultrasound Med Biol. 1978; 4:317–27.
10. Salustri A, Roelandt JR. Ultrasonic three-dimensional reconstruction of the heart. Ultrasound Med Biol. 1995; 21:281–93.
11. von Ramm OT, Smith SW. Real time volumetric ultrasound imaging system. J Digit Imaging. 1990; 3:261–6.
12. Mondelli JA, Di Luzio S, Nagaraj A, Kane BJ, Smulevitz B, Nagaraj AV, Greene R, McPherson DD, Rigolin VH. The validation of volumetric real-time 3-dimensional echocardiography for the determination of left ventricular function. J Am Soc Echocardiogr. 2001; 14:994–1000.
13. Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol. 2004; 44:878–86.
14. Otterstad JE, Froeland G, St John SM, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997; 18:507–13.
15. Otterstad JE. Measuring left ventricular volume and ejection fraction with the biplane Simpson's method. Heart. 2002; 88:559–60.
16. Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995; 8:221–8.
17. Jenkins C, Chan J, Hanekom L, Marwick TH. Accuracy and feasibility of online 3-dimensional echocardiography for measurement of left ventricular parameters. J Am Soc Echocardiogr. 2006; 19:1119–28.
18. Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO. ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr. 2003; 16:1091–110.
19. Hare JL, Jenkins C, Nakatani S, Ogawa A, Yu CM, Marwick TH. Feasibility and clinical decision-making with 3D echocardiography in routine practice. Heart. 2008; 94:440–5.
20. Mor-Avi V, Jenkins C, Kuhl HP, Nesser HJ, Marwick T, Franke A, Ebner C, Freed BH, Steringer-Mascherbauer R, Pollard H, Weinert L, Niel J, Sugeng L, Lang RM. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovasc Imaging. 2008; 1:413–23.
21. Marsan NA, Bleeker GB, Ypenburg C, Van Bommel RJ, Ghio S, Van de Veire NR, Delgado V, Holman ER, van der Wall EE, Schalij MJ, Bax JJ. Real-time three-dimensional echocardiography as a novel approach to assess left ventricular and left atrium reverse remodeling and to predict response to cardiac resynchronization therapy. Heart Rhythm. 2008; 5:1257–64.
22. Kapetanakis S, Bhan A, Murgatroyd F, Kearney MT, Gall N, Zhang Q, Yu CM, Monaghan MJ. Real-time 3D echo in patient selection for cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2011; 4:16–26.
23. Chen X, Sun D, Yang J, Feng W, Gu T, Zhang Z, Xiu Z, Tang L, Ma C, Wang X, Cheng Y, Li N, Liu S. Preoperative assessment of mitral valve prolapse and chordae rupture using real time three-dimensional transesophageal echocardiography. Echocardiography. 2011; 28:1003–10.
24. Pepi M, Tamborini G, Maltagliati A, Galli CA, Sisillo E, Salvi L, Naliato M, Porqueddu M, Parolari A, Zanobini M, Alamanni F. Head-to-head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J Am Coll Cardiol. 2006; 48:2524–30.
25. Schlosshan D, Aggarwal G, Mathur G, Allan R, Cranney G. Real-time 3D transesophageal echocardiography for the evaluation of rheumatic mitral stenosis. JACC Cardiovasc Imaging. 2011; 4:580–8.
26. Kahlert P, Plicht B, Schenk IM, Janosi RA, Erbel R, Buck T. Direct assessment of size and shape of noncircular vena contracta area in functional versus organic mitral regurgitation using real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2008; 21:912–21.
27. Eng MH, Salcedo EE, Quaife RA, Carroll JD. Implementation of real time three-dimensional transesophageal echocardiography in percutaneous mitral balloon valvuloplasty and structural heart disease interventions. Echocardiography. 2009; 26:958–66.
28. Maffessanti F, Marsan NA, Tamborini G, Sugeng L, Caiani EG, Gripari P, Alamanni F, Jeevanandam V, Lang RM, Pepi M. Quantitative analysis of mitral valve apparatus in mitral valve prolapse before and after annuloplasty: a three-dimensional intraoperative transesophageal study. J Am Soc Echocardiogr. 2011; 24:405–13.
29. Fattouch K, Castrovinci S, Murana G, Novo G, Caccamo G, Bertolino EC, Sampognaro R, Novo S, Ruvolo G, Lancellotti P. Multiplane two-dimensional versus real time three-dimensional transesophageal echocardiography in ischemic mitral regurgitation. Echocardiography. 2011; 28:1125–32.
30. Kronzon I, Sugeng L, Perk G, Hirsh D, Weinert L, Garcia Fernandez MA, Lang RM. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. J Am Coll Cardiol. 2009; 53:1543–7.
31. Becerra JM, Almeria C, de Isla LP, Zamorano J. Usefulness of 3D transoesophageal echocardiography for guiding wires and closure devices in mitral perivalvular leaks. Eur J Echocardiogr. 2009; 10:979–81.
32. Seliem MA, Fedec A, Cohen MS, Ewing S, Farrell PE Jr, Rychik J, Schultz AH, Gaynor JW, Spray TL. Real-time 3-dimensional echocardiographic imaging of congenital heart disease using matrix-array technology: freehand real-time scanning adds instant morphologic details not well delineated by conventional 2-dimensional imaging. J Am Soc Echocardiogr. 2006; 19:121–9.
33. Taniguchi M, Akagi T, Watanabe N, Okamoto Y, Nakagawa K, Kijima Y, Toh N, Ohtsuki S, Kusano K, Sano S. Application of real-time three-dimensional transesophageal echocardiography using a matrix array probe for transcatheter closure of atrial septal defect. J Am Soc Echocardiogr. 2009; 22:1114–20.
34. Kijima Y, Taniguchi M, Akagi T, Nakagawa K, Kusano K, Ito H, Sano S. Torn atrial septum during transcatheter closure of atrial septal defect visualized by real-time three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr. 2010; 23:1222–8.
35. Balzer J, Kuhl H, Rassaf T, Hoffmann R, Schauerte P, Kelm M, Franke A. Real-time transesophageal three-dimensional echocardiography for guidance of percutaneous cardiac interventions: first experience. Clin Res Cardiol. 2008; 97:565–74.
36. Zhang YL, Wang M, Xue XP, Hu B. The value of live three-dimensional echocardiography in an adult patient with aortico-left ventricular tunnel. Echocardiography. 2011. Oct 4. [Epub ahead of print]
37. Khairnar P, Hsiung MC, Mishra S, Nanda NC, Daly DD Jr, Nayyar G, Patel A, Mishra J, Chuang YC, Tsai SK, Yin WH, Wei J. The ability of live three-dimensional transesophageal echocardiography to evaluate the attachment site of intracardiac tumors. Echocardiography. 2011; 28:1041–5.
38. Perk G, Biner S, Kronzon I, Saric M, Chinitz L, Thompson K, Shiota T, Hussani A, Lang R, Siegel R, Kar S. Catheter-based left atrial appendage occlusion procedure: role of echocardiography. Eur J Echocardiogr. 2011. Sep 8. [Epub ahead of print]
39. Landmesser U, Holmes DR, Jr. Left atrial appendage closure: a percutaneous transcatheter approach for stroke prevention in atrial fibrillation. Eur Heart J. 2011. Oct 31. [Epub ahead of print]
40. Jenkins C, Monaghan M, Shirali G, Guraraja R, Marwick TH. An intensive interactive course for 3D echocardiography: is 'crop till you drop' an effective learning strategy? Eur J Echocardiogr. 2008; 9:373–80.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528


