The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Review Article
Renal Denervation in Resistant Hypertension: An Emerging Novel Therapy
Volume 2, Apr 2013
C. Venkata S. Ram, MD, MACP, FACC, Mohsin Wali, MD, FACC; Hyderabad, New Delhi, India
J Clin Prev Cardiol. 2013;2(2):73:83
Background
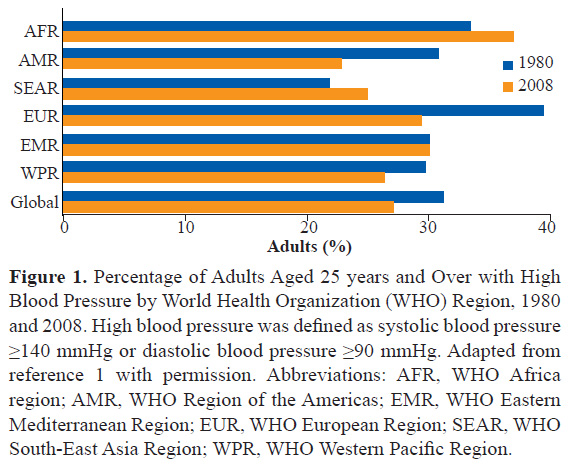
Hypertension is a significant and global disease burden. Overall, approximately 20-40% of patients around the world have high blood pressure (BP)(Figure 1) (1). Hypertension is also a major risk factor for the occurrence of cardiovascular disease in India. Hypertension prevalence is not only increasing steadily in India but is responsible for excessive mortality; uncontrolled hypertension imposes significant disease burden (2). India is witnessing an unprecedented surge in cardiovascular disease (3). Therefore, there should be a focused approach to control hypertension effectively across the entire country.
Hypertension is said to be resistant when, at a minimum, the patient has uncontrolled BP despite the use of 3 or more antihypertensive drugs at optimal doses, including a diuretic (4). The incidence of resistant hypertension has been estimated at 0.7 cases per 100 person-years of follow-up (4). Resistant hypertension prevalence has been examined in numerous observational studies and USAsurveys, which have generally reported rates of 12 to 15% (4). Higher prevalence values have been found in clinical trials. Resistant hypertension was found in 35% of previously untreated and 50% of previously treated patients at 5 years of follow-up, up to 50% after 5 years of treatment, and 25-28% at a mean follow-up of 3 years (4). However, study enrollment criteria and medication regimens may have resulted in an incidence that is not reflective of the general population of people with hypertension. Taking into account observational and clinical trial data, a prevalence of 15-30% of adults with hypertension has been suggested as a reasonable estimate (4). Resistant hypertension is quite common in India. Surveys done in India have emphasized the adverse impact of hypertension on the community (5).
Renal denervation (RDN) therapy is being studied and widely applied as an experimental tool for managing resistant hypertension (6). An early application of sympathetic denervation was radical surgical sympathectomy, which lowered BP substantially butresulted in significant mortality and morbidity including intolerable postural hypotension. Surgical sympathectomy because of serious side effects was therefore abandoned in the 1970s. Subsequent research on the role of the sympathetic nervous system (SNS) in hypertension, especially with the study of microneurography and norepinephrine spillover in humans, led to the adoption of catheter-based radio frequency (RF) ablation of the renal nerves (6,7). This review summarizes the current clinical research and status on RDN in resistant hypertension and examines new directions for this emerging novel therapeutic approach.
Etiologic Factors and Patient Characteristics in Resistant Hypertension
Certain factors are linked to resistant hypertension such as concomitant conditions, genetic markers, and distinguishingpatient characteristics (8). Concomitant conditions such as hyperaldosteronism, salt sensitivity, obstructive sleep apnea (OSA), and obesity have been implicated in resistant hypertension. The AGT235 T, or angiotensinogen, allele has been associated with and determined to be an independent risk factor for resistant hypertension. Other patient characteristics associated with resistant hypertension include old age, high baseline BP, chronic kidney disease (CKD), left ventricular (LV) hypertrophy, diabetes, obesity, female sex, race, and excessive sodium intake (8). A large review concerning medication non-compliance calculated that <65% of patients being treated for hypertension were compliant with their medications. The same review calculated that only 69% of patients persistently took their medications over many years (9).
Prospective studies in India show that hypertension is not controlled in a majority of people living in urban areas of the country (10). Unfortunately, in India, the urban – rural difference in hypertension prevalence is disappearing as demonstrated by epidemiological observations (11).
Physiological Rationale of Renal Denervation (RDN) in Resistant Hypertension
Renal afferent and efferent nerve output affects SNS activity and can contribute to high BP (Figure 2) (12). Ischemia, hypoxia, and other conditions can increase renal afferent activity, which has a direct influence on systemic adrenergic drive via modulation of posterior hypothalamic activity. These processes cause increased systemic vascular resistance that in turn lead to hypertension. Stimulating the renal efferent nerves increases the release of renin and sodium reabsorption and decreases renal blood flow. The latter process initiates the renin-angiotensin-aldosterone system (12). The consequences of heightened sympathetic tone mediated by the kidney are possible increases in morbidity and mortality rates (13).
.jpg)
Interruption of the connection between the brain sympathetic activity and the kidney has been seen in preclinical trials to have an influence on BP via obliterating the systemic consequences of sympathetic over-activity. The increase in sympathetic tone can be blunted via renal afferent nerve disruption (14-21). The consequences of increased efferent sympathetic activity can also be reversed via disruption of renal nerves (13,22). Therefore, RDN is likely to be a valuable therapeutic option in the treatment of hypertension and comorbidities by offsetting the adverse impact of the SNS on cardiovascular function.
Data Supporting the Potential Scope for RDN to Treat Hypertension
In addition to the aforementioned research based on microneurography and norepinephrine spillover (6,7), nephrectomy in patients with end-stage renal disease(ESRD) has been shown to lower the BP presumably by removing the contribution of renal nerves to hypertension.
Onesti et al. reported impressive BP responses to bilateral nephrectomy in 29 patients with ESRD on maintenance hemodialysis and 3 patients following renal transplantation (23). In patients with hypertension, bilateral nephrectomy resulted in a consistent reduction in BP and total systemic vascular resistance. In contrast, those patients who were originally normotensive remained so, with no change in BP or systemic vascular resistance. In patients with impaired systolic cardiac performance, nephrectomy resulted in improved cardiac output. In three patients, BP reduction was delayed up to 3 months, which is consistent with the data reported by Hampers in a study using bilateral nephrectomy to treat hypertension (24). These observations suggest that removing the neural factors in the kidney may lower the systemic BP levels.

RDN treatment was not associated with any significant complications. One patient experienced renal artery dissection upon placement of the catheter before RF energy was delivered; the dissection was treated with renal artery stent without any subsequent complications. Three other patients developed catheter access site complications; all were treated without any subsequent complication. At 6 months follow-up, one patient was found to have had a progression of a preexisting renal artery stenosis in the proximal portion of the renal artery which was successfully stented. There was one new moderate stenosis identified that was not hemodynamically significant and required no treatment. There were no other new reports of vascular complications reported at 24 (n=104) and 36 (n=34) month analysis (32). There was no evidence of renal impairment and renal function was maintained as measured by estimated glomerular filtration rate (eGFR) and creatinine through 36 months follow-up. No patients were hospitalized due to hypotension. The overall results of this pilot study are important because it demonstrated for the first time a significant, safe and persistent BP lowering effect in a series of patients with the use of the Symplicity catheter to treat resistant hypertension.
Symplicity HTN-2 Trial

Renal denervation (RDN) therapy is being studied and widely applied as an experimental tool for managing resistant hypertension (6). An early application of sympathetic denervation was radical surgical sympathectomy, which lowered BP substantially butresulted in significant mortality and morbidity including intolerable postural hypotension. Surgical sympathectomy because of serious side effects was therefore abandoned in the 1970s. Subsequent research on the role of the sympathetic nervous system (SNS) in hypertension, especially with the study of microneurography and norepinephrine spillover in humans, led to the adoption of catheter-based radio frequency (RF) ablation of the renal nerves (6,7). This review summarizes the current clinical research and status on RDN in resistant hypertension and examines new directions for this emerging novel therapeutic approach.
Etiologic Factors and Patient Characteristics in Resistant Hypertension
Certain factors are linked to resistant hypertension such as concomitant conditions, genetic markers, and distinguishingpatient characteristics (8). Concomitant conditions such as hyperaldosteronism, salt sensitivity, obstructive sleep apnea (OSA), and obesity have been implicated in resistant hypertension. The AGT235 T, or angiotensinogen, allele has been associated with and determined to be an independent risk factor for resistant hypertension. Other patient characteristics associated with resistant hypertension include old age, high baseline BP, chronic kidney disease (CKD), left ventricular (LV) hypertrophy, diabetes, obesity, female sex, race, and excessive sodium intake (8). A large review concerning medication non-compliance calculated that <65% of patients being treated for hypertension were compliant with their medications. The same review calculated that only 69% of patients persistently took their medications over many years (9).
Prospective studies in India show that hypertension is not controlled in a majority of people living in urban areas of the country (10). Unfortunately, in India, the urban – rural difference in hypertension prevalence is disappearing as demonstrated by epidemiological observations (11).
Physiological Rationale of Renal Denervation (RDN) in Resistant Hypertension
Renal afferent and efferent nerve output affects SNS activity and can contribute to high BP (Figure 2) (12). Ischemia, hypoxia, and other conditions can increase renal afferent activity, which has a direct influence on systemic adrenergic drive via modulation of posterior hypothalamic activity. These processes cause increased systemic vascular resistance that in turn lead to hypertension. Stimulating the renal efferent nerves increases the release of renin and sodium reabsorption and decreases renal blood flow. The latter process initiates the renin-angiotensin-aldosterone system (12). The consequences of heightened sympathetic tone mediated by the kidney are possible increases in morbidity and mortality rates (13).
.jpg)
Interruption of the connection between the brain sympathetic activity and the kidney has been seen in preclinical trials to have an influence on BP via obliterating the systemic consequences of sympathetic over-activity. The increase in sympathetic tone can be blunted via renal afferent nerve disruption (14-21). The consequences of increased efferent sympathetic activity can also be reversed via disruption of renal nerves (13,22). Therefore, RDN is likely to be a valuable therapeutic option in the treatment of hypertension and comorbidities by offsetting the adverse impact of the SNS on cardiovascular function.
Data Supporting the Potential Scope for RDN to Treat Hypertension
In addition to the aforementioned research based on microneurography and norepinephrine spillover (6,7), nephrectomy in patients with end-stage renal disease(ESRD) has been shown to lower the BP presumably by removing the contribution of renal nerves to hypertension.
Onesti et al. reported impressive BP responses to bilateral nephrectomy in 29 patients with ESRD on maintenance hemodialysis and 3 patients following renal transplantation (23). In patients with hypertension, bilateral nephrectomy resulted in a consistent reduction in BP and total systemic vascular resistance. In contrast, those patients who were originally normotensive remained so, with no change in BP or systemic vascular resistance. In patients with impaired systolic cardiac performance, nephrectomy resulted in improved cardiac output. In three patients, BP reduction was delayed up to 3 months, which is consistent with the data reported by Hampers in a study using bilateral nephrectomy to treat hypertension (24). These observations suggest that removing the neural factors in the kidney may lower the systemic BP levels.
Curtis et al. have documented the results of elective surgical bilateral native kidney nephrectomy post allograft renal transplant in 10 patients, resolving hypertension irrespective ofrenin levels (25). These findings are entirely consistent with the hypothesis that renal sympathetic traffic mediates hypertension in ESRD via intact native kidney(s).
The therapeutic utility of denervation of the native kidney in ESRD patients with hypertension and LV hypertrophy was recently suggested following the documentation of a regression of left LV mass following bilateral nephrectomy (26). The extent of left LV regression was nearly twice that seen in prior pharmacologic efforts to induce reduction of heart mass, and occurred to a similar extent in the Losartan Intervention for Endpoint Reduction (LIFE) trial after 48 months (27) and compares favorably to the LV mass reduction of 27 g at 9 months seen in patients taking inhibitors of the renin-angiotensin system (28). Surgical removal of the non-functioning kidneys achieved more rapid and effective regression of LV hypertrophy, than pharmacologic blockade of the renin-angiotensin-aldosterone system and thus implies the mechanism of benefit of the nephrectomy was at least due in part to interference with the renal sympathetic tone.
Clinical Results of RDN to Treat Resistant Hypertension
The earliest clinical data on using catheter-based RFRDN in treating patients with resistant hypertension was published in 2009 (29). Since then, RDN therapy has been used in treating many other patients and several case reports and a significant number of case series/trials describing its benefit in treating resistant hypertension in more than 800 patients.
Symplicity HTN-1 Trial
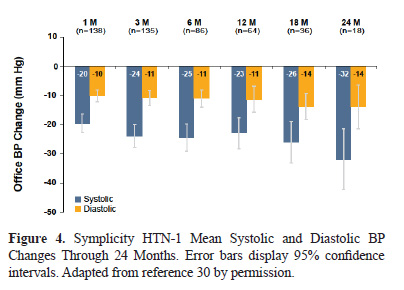
Symplicity HTN-1 wasa pilot trial which utilized the Symplicity™ Renal Denervation System (Figure 3 and 4) for treating patients with resistant hypertension (29,30). A total of 153 resistant hypertension patients with baseline systolic BP ≥160 mmHg were treated in this single-arm trial and 36 month follow-up data is available. At the primary endpoint, 6 months post-procedure, the investigators reported a -22/-10 mm Hg change in systolic BP from baseline. The magnitude of clinical response was sustained through 36 months (31). The percentage of patients responding to RDN treatment with greater than a 10 mmHg reduction in systolic BP at 1 month was 69% and at 36 months was 94%, indicating continued fall in BP over time.


RDN treatment was not associated with any significant complications. One patient experienced renal artery dissection upon placement of the catheter before RF energy was delivered; the dissection was treated with renal artery stent without any subsequent complications. Three other patients developed catheter access site complications; all were treated without any subsequent complication. At 6 months follow-up, one patient was found to have had a progression of a preexisting renal artery stenosis in the proximal portion of the renal artery which was successfully stented. There was one new moderate stenosis identified that was not hemodynamically significant and required no treatment. There were no other new reports of vascular complications reported at 24 (n=104) and 36 (n=34) month analysis (32). There was no evidence of renal impairment and renal function was maintained as measured by estimated glomerular filtration rate (eGFR) and creatinine through 36 months follow-up. No patients were hospitalized due to hypotension. The overall results of this pilot study are important because it demonstrated for the first time a significant, safe and persistent BP lowering effect in a series of patients with the use of the Symplicity catheter to treat resistant hypertension.
Symplicity HTN-2 Trial
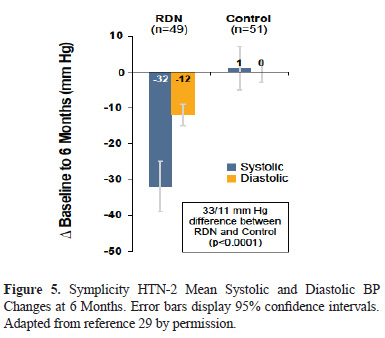
The Symplicity HTN-2 randomized controlled trial (Figure 5) was conducted in a population of patients with treatment-resistant hypertension. The efficacy of RDN was compared with conventional medical therapy (33,34). At the 6 month primary end-point, the investigators reported a -32/-12 mmHg change in office systolic BP in the RDN group compared to a +1/0 change in the control group (p<0.0001). After 6 months control patients could choose to crossover to receive RDN therapy. Consistent BP lowering effects were observedin both groups and have been sustained in follow-up (33).
There were no serious device-related or procedure-related complications observed at primary endpoint. The occurrence of adverse events through 6 months did not differ between treatment groups. The renal arteries of treated patients were imaged at 6 months and a single patient was reported to have had possible progression of an underlying atherosclerotic lesion, but required no treatment. At 24 months follow-up, there were no clinially significant changes in eGFR compared to preprocedure values, and no renal vascular events were reported. Importantly, the results of Symplicity HTN-2 reaffirmed and extended the results of the Symplicity HTN-1 trial in a randomized controlled trial and showed a significant improvement in hypertension compared with optimal medical management.
Other Potential Clinical Benefits of RDN

Other Potential Clinical Benefits of RDN
Results from clinical studies of RDN in resistant hypertension and other conditions have suggested the therapy may be useful in several other indications. These results are preliminary and require further assessment before they can be generally recommended.
Moderate Resistant Hypertension (Baseline SBP 140-160 mmHg)
The effectiveness of RDN treatment with the Symplicity catheter for resistant hypertension subjects with baseline systolic BP of 140 – 160 mmHg has been investigated. A study of twenty consecutive subjects with a mean systolic BP of 148.4 mmHg demonstrated systolic BP reductions of 13.1/5.0 ± 13.6/8.3 mmHg (p<0.01) at 6 months (35). Four patients were able to reduce antihypertensive medications prior to their 3 month visit. At 6 month follow-up, mean ambulatory 24h BP was reduced by 11.3/4.1 ± 8.6/7.3 mmHg (p<0.01). This study suggests that less severe forms of resistant hypertension may benefit from RDN as a therapeutic alternative to drug therapy.
Arterial Stiffness and Central Hemodynamics
Another study performed RDN with the Symplicity catheter in 110 patients with resistant hypertension and evaluated the effect of treatment on arterial stiffness and central hemodynamics in addition to evaluating the improvement in BP response. Mean office BP reduction was 25/7 mm Hg at 6 months (p<0.001)(36). Additionally, the study demonstrateda significant reduction in carotid to femoral pulse wave velocity from 11.6± 3.2 m/s to 9.6± 3.1 m/s at 6-months (p<0.001) and cardiac work load following RDN therapy.
In another study, RDN was performed in 21 patients with systolic peripheral BP >150 mm Hg and patients were evaluated similarly to the above described study (37). After 6 months, systolic BP was reduced by 6.1% (p<0.05) while central systolic pressure was reduced by 7.0% (p<0.05). Subgroup analysis showed that in responders, peripheral systolic BP was reduced by 16.1% (p<0.01) while central systolic pressure was reduced by 18.3% (p<0.01). Arterial stiffness improved significantly. Aortic augmentation index (AIx) improved by 9.5% (p<0.05). In responders, AIx improved by 19.2% (p<0.02). Pulse wave velocity was high at baseline (10.8 m⁄ s) and improved by 10.4% (p<0.05). These important findings suggest that RDN therapy not only lower the BP levels but improves the cardiovascular hemodynamics in patients with hypertension.
RDN and Chronic kidney disease (CKD)
A recent study explored the effects of RDN therapy on changes in renal resistive index (RRI), urinary albumin excretion rate (UAE), and renal function in 100 patients with resistant hypertension in a controlled trial (38). All patients hadan eGFR >45 mL/min/1.73 m² at baseline. At 6 months follow-up, mean office BP was reduced significantly in the treatment group vs. controls not receiving RDN therapy. RRI decreased significantly from 0.691 ± 0.01 at baseline to 0.674 ± 0.01 and 0.670 ± 0.01 (p<0.037/0.017) at 3 and 6 month follow-up, and mean cystatin C glomerular filtration rate (GFR) remained unchanged. Importantly, the investigators observed that the number of patients with microalbuminuria or macroalbuminuria decreased. These findings confirmed the importance of increased sympathetic tone in hypertension and associated renal disease, addressed concerns regarding the effect of RDN on renal function, as well as showed that RDN may have a reno-protective effect.
Another study explored the effects of RDN therapy in patients with reduced renal function (39). Hering et al. treated 15 patients diagnosed with CKD stages 3 – 5. In this study, eGFR remained unchanged after the procedure and no significant postprocedural changes occurred in effective renal plasma flow. No statistically significant differences in postprocedural serum and urine biochemistries were observed. Overall, it was demonstrated that in this subgroup of patients renal function did not deteriorate and significant BP lowering efficacy was achieved safely.
A more recent study analyzedconcentrations of 2 biomarkers of acute kidney injury (urinary neutrophil gelatinase-associated lipocalin [NGAL] and kidney injury molecule-1 [KIM-1]), in addition to serum creatinine, BUN, and eGFR, in 62 patients undergoing RDN (40). Eight of 62 patients had a baseline eGFR<45 ml/min/1.73 m2. A significant systolic BP reduction was observed at both 1 and 3-months follow-up. Measurement of urinary NGAL and KIM-1 concentrations showed no significant changes 24 and 48 h after RDN compared with baseline as well as at the 3-month follow-up. In addition, measurements of eGFR and the serum creatinine levels showed no significant differences at 1 and 3 months follow-up. Even in 8 patients who had an impaired renal function at baseline, no alterations regarding KIM-1 and NGAL levels either in the early postprocedural period or after 3 months were observed. The results of this study confirm that RDN does not have an adverse effect on the kidney function.
Effects of RDN on Heart Rate and Other Physiological Responses
Effects of RDN on Heart Rate and Other Physiological Responses
The effect of RDN on heart rate was assessed in 14 patients with resistant hypertension (41). Holter electrocardiographic recordings were analyzed for time and frequency domain heart rate variability parameters before and 3 months after percutaneous renal sympathetic denervation. One month and 3 months after RDN treatment, office BP was reduced by 16/4 ± 6/5 mmHg and 23/9 ± 8/6 mmHg (baseline of 170/94 ± 18/14 mmHg, p<0.05). Three months after RDN treatment, patients showed improved cardiovascular dynamics and cardiac autonomic tone indicating a potential protective effect of the RDN procedure on the heart in patients with resistant hypertension.
Another pivotal study, in similar fashion aimed to investigate the effects of RDN on heart rate and other electrocardiographic parameters on 136 patients with resistant hypertension (42). After 3 months and 6 months, systolic BP was reduced by 25.5 ± 2.4 mmHg (p<0.0001) and 28.1 ± 3 mmHg (p<0.0001). Heart rate at baseline was 66.1 ± 1 beats per minute and was reduced by 2.6 ± 0.8 beats per minute after 3 months (p=0.001) and 2.1 ± 1.1 beats per minute after 6 months (p=0.046). The PR interval was prolonged by 11.3 ± 2.5 ms (p<0.0001) and 10.3 ± 2.5 ms (p<0.0001) at 3 and 6 months after RDN, respectively. The study confirms the improved cardiovascular dynamics after RDN in addition to BP control. Thus one can hope for overall cardiovascular improvement as a result of RDN therapy beyond BP control.
The effects of RDN therapy on physiologic responses to physical stress (including heart rate, cardiac output, BP, and ventilation) have been investigated on 46 patients in a substudy of Symplicity HTN-2 (43). It was observed that BP at maximal exercise was reduced in the treated group compared to controls, without compromise of chronotropic competence or the work performed. RDN therapy was shown to decrease the heart rate at rest and it’s as well. In addition to confirming the beneficial effects of RDN in BP control, this study showed that RDN reduced BP at rest, during physical exercise, and at recovery in the study population without affecting the physiological cardiopulmonary response. These observations confirm the physiological and pathological interrelationship between the SNS, cardiovascular system, BP regulation and the beneficial effects of RDN therapy.
Cardiac Function and Heart Failure
Recently it was shown for the first time that RDN therapy reduced LV mass and improved diastolic function in patients with resistant hypertension (44). After RDN, the mean interventricular septum thickness and LV mass index decreased as well as the mitral valve lateral E/E=1 and 6 months, indicating reduction of LV filling pressures. Also, isovolumic relaxation time shortened and ejection fraction significantly increased after RDN. These results indicated that RDN therapy, in addition to lowering peripheral BP, significantly reduced LV mass and improved diastolic function in patients with resistant hypertension. Thus, RDN therapy produces physiologic and structural improvements in patients with resistant hypertension.
Metabolic Parameters and Glycemic Control
Mahfoud et al. showed improvements in insulin activity and glucose metabolism after RDN treatment of 50 patients with uncontrolled hypertension (45) (Figure 6). Three months after RDN, mean fasting glucose, insulin, C-peptide levels, and homeostasis model assessment–insulin resistance decreased significantly. Additionally, mean 2-hour glucose levels during oral glucose tolerance test (OGTT) were reduced by 27 mg/dL (p<0.012). There were no significant changes in BP or metabolic markers in the control group. This study for the first time demonstrated that denervation of the renal nerves has the potential to improve glucose metabolism in addition to BP control in patients with uncontrolled hypertension.


Obstructive Sleep Apnea (OSA)
The results of RDN therapy on 10 patients with resistant hypertension and OSA further document the BP and metabolic benefits of RDN therapy (46). Similarly significantdecreases in BP, plasma glucose concentrations, hemoglobin A1C level, as well as decreases in apnea-hypopnea index (AHI) at 6 months after RDN therapy were observed. The decrease in the AHI suggests that RDN therapy may have potential benefit in patients with OSA.
Safety of RDN
Renal nerve ablation using the Symplicity system isperformed with smaller electrodes that deliver onlylow frequency ablation (~8 W), minimizing the risk for vascular injury (47). Preclinical animal studies conducted byfocused on long-term vascular safety following RF application to the renal arteries using the Symplicity system in a Yorkshire domestic pig model. This study involved 7 animals and utilized RF catheters and generators identical to those utilized in the Symplicity HTN-1 and HTN-2 studies (48). Vascular histopathology performed at 6 months revealed that treatment did not result in any damage to the renal arteries, or other tissues. No stenosis or significant luminal narrowing was seen in any treated renal artery at 6 months either on angiography or on histology sections. No inflammatory component was seen in 6-month histopathology assessment, consistent with complete healing process by that time point; given what we know about vascular biology, it is unlikely that the vascular abnormalities would develop beyond this time point. Unlike with stents or other implanted foreign devices, there is no indwelling source of irritation to induce inflammatory changes with the Symplicity device. Given the absence of pathology seen 6 months after denervation, late intimal or smooth muscle hyperplasia, thrombosis, aneurysmal dilatation, and other vascular events beyond 6 months would not be expected (48).
In another experimental study,animals were assigned to either the acute (n = 6), subacute (10-day follow-up, n = 6) or control (untreated, n = 2) group. Angiography and optical coherence tomography (OCT) were used to assess histopathology and immunohistochemistry of the renal arteries and kidneys. Transmural tissue coagulation and loss of endothelium coating resulted from local thrombus formation after RF use. Re-endothialization was nearly complete at 10 days. Fibrotic tissue and the advential layer replaced mural wall damage, indicating vasculogenesis. The subacute group’s remaining autonomic nerve fascicles had an increase in vacuolic degeneration and decrease in neurofilament protein immunostaining. There were no kidney abnormalities, and blood test results were normal (49).
In humans, the best available evidence on the vascular safety of RF ablation of renal arteries is from the Symplicity HTN-1 and HTN-2 trials (29,30,33,34). In the Symplicity HTN-1 trial short-term follow-up angiography was performed in the first 20 patients revealing no evidence of renal artery stenosis or abnormalities in treated arteries. In 81 patients with 6-month renal imaging, no new irregularities or stenosis at any treatment site were identified (29,30). At 18 months follow-up, one progression of a preexisting stenosis at a site distinct from the RF treatment sites and one new moderate stenosis at an RF treatment site was observed; this was not hemodynamically relevant requiring no treatment. No other vascular pathology has been reported in patients who have reached 24 and 36 months follow-up (31). In Symplicity HTN-2, of 43 patients who underwent RDN therapy and had renal imaging at 6 months, there was only one patient who had a possible progression of an underlying atherosclerotic lesion at a site distant from the RF treatment sites; no intervention was needed (33,34). At 18 months follow-up, no new vascularabnormalitywas reported(50).
Renal Function
The human kidney transplant experience (51,52) and the RDN experience using the Symplicity Renal Denervation System (29,30,33,34,38,39) have demonstrated that RDN does not have untoward side-effects or interfere with the ability of the kidney to maintain appropriate electrolyte/salt and volume homeostasis. In the randomized controlled trial of Symplicity HTN-2, RDN treatment and control arms demonstrated no statistically significant differences in eGFR, as well as serum creatinine and serum cystatin C levels at 6 and 12 months follow-up (33,34). The renal safety results from these studies have been confirmed in a 100 patient case control study where the mean cystatin C glomerular filtration rate and UAE remained unchanged after RDN (38). Additionally, RDN therapy with the Symplicity catheter has been performed safely with subjects with moderate to severe CKD Class 3b and 4 (Stage 3b = GFR 30-44 mL/min/1.73 m², Stage 4 = 15-29 mL/min/1.73 m²) (39). In this study of 15 subjects, subjects had a significant BP reduction, no change in eGFR and a trend toward reduction in albuminuria and BNP levels was noticed.
Orthostatic Hypotension
The loss of orthostatic response observed in early trials of crude surgical sympathectomy was attributed partially to the removal of splanchnic nerves during surgery causing disruption in the autonomic vasoconstrictor response which does not occur with current RDN therapy. In the current literature, there are no cases of orthostatic hypotension reported after RDN therapy (29,30,33-46,53-56).
Additionally, a recent study evaluated patients through tilt-table testing after RDN therapy and demonstrated that none of the subjects developed orthostatic dysfunction (57). After tilting, the maximal reduction of BP compared with that measured in the supine position was not altered after RDN (Δ max SBP: –32 ± 5 vs. –27 ± 5 mmHg, Δ max DBP: –14 ± 2 vs. –13 ± 3 mmHg; p>0.05). In addition, the minimal BP during the 20 min tilting period also demonstrated no different following denervation whereas mean HR during the 20 min tilting period was significantly reduced by 4.9 beats/min (p<0.05). These observations suggest that after RDN therapy patients can still maintain their physiological responses to change in position and maintain orthostatic regulation. Furthermore, catheter-based RDN therapy directly targets only nerves adjacent to the renal arteries preventing damage to more distant nerves, such as the splanchnic nerves, thus allowing for intact orthostatic regulation.
Cardiac Function and Physiological Responses
The effects of RDN therapy on physiologic responses to physical stress including heart rate, cardiac output, BP, and ventilation have been investigated on 46 patients in a substudy of Symplicity HTN-2 (43). It was observed that BP at maximal exercise was reduced in the treated group compared to controls, without compromise of chronotropic competence or the work performed. RDN was shown to decrease heart rate at rest and improve heart rate recovery as well. In addition to confirming the beneficial effects of RDN in BP control, this study shows that RDN reduces BP at rest, during physical exercise, and at recovery without affecting the physiological cardiopulmonary response to exercise. Adverse event reporting has not demonstrated elevated rates of infection or stress intolerance from the Symplicity clinical studies of over 200 patients with follow-up to three years in Symplicity HTN-1 and to two years in Symplicity HTN-2.
Reinnervation of Kidneys
Regrowth of nerves following surgical transection has been studied in the cardiac and renal transplant experience in both animals and humans. Efferent renal innervation after surgical denervation can be demonstrated after 3–4 months in both rats and dogs (58,59). In humans, following surgical transection of the renal nerves, histological studies of transplanted kidneys showed that axonal regeneration begins as early as 28 days after transplantation and appeared to be complete by 8–12 months (60,61). However, while regenerating axons could be traced to the nerves accompanying the interlobular arteries, the axon population did not reach the numbers observed in normal nerves of comparable size. These findings suggest that anatomic reinnervation of the kidneys may occur as early as in 8 months however; the anatomic connections appears not be associated with normal function. Therefore, nerves that regrow do not appear to be functional (60,61). The functional characteristics of transplanted kidneys beyond two years were examined and supersensitivity to circulating noradrenaline and an inadequate response to lower body negative pressure were identified, also supporting the hypothesis that functional integrity was retained (62). In contrast to efferent nerves, the afferent nerves do not seem to have the capacity to regrow in humans (63).In Symplicity HTN-1 and HTN-2 studies, no loss of antihypertensive response was evident with follow-ups up to three years in Symplicity HTN-1 study and to two years in the Symplicity HTN-2 study. Currently, the data from these studies provide the longest published follow-up time in patients treated with catheter-based RFRDN. These data demonstrate the long-term durability of the procedure and the lack of functional re-innevation following RDN using the Symplicity RDN procedure.
Clinical Data with the Use of RDN Devices other than Symplicity Catheter
The above discussed data was from trials and case series that utilized Symplicity Renal Denervation System. Other than these studies clinical data utilizing other RF (64-67), ultrasound (68), and cryoenergy (69) RDN catheters have been published. Currently, data on these devices is very scarce and a meaningful evaluation on their safety and efficacy is not possible as further clinical trials are required. The largest body of clinical evidencefrom controlled studies so far has been with the Simplicity RDN system.
Future Prospects for RDN Therapy
RF and other RDN technologies (e.g., microwave) are still under development. Clinical results of RDN therapy in resistant hypertension are compelling, but there remain some unresolved issues with the therapy. At present, it is unclear how energy dosage, location and distribution of ablation, and penetration depth affect the safety and efficacy in the long run (70). It is also unclear why some patients have a delayed response to RDN and whichpredictors of response are valuable in tailoring the therapy (6,70,71). It is possible that patients with less severe hypertension may also benefit from RDN,but trials investigating the therapy in this population would be challenging to design and implement (70). If RDN therapyis successful in such patients, however,this could reduce or eliminate the need for antihypertensive medications (47). Other possible indications for RDN that have been identified include heart failure, ESRD, obesity, insulin resistance, OSA, cardiorenal, and hepatorenal syndromes (47).
RDN therapy represents an unprecedented novel approach to tackle the complex problem of resistant hypertension and other related disorders. Further studies and observations are needed toassign a precise role of RDN therapy in the prevention and treatment of cardiovascular diseases in modern clinical practice.
Financial Disclosures
Dr. Ram is an investigator for the Symplicity HTN-3 Trial sponsored by Medtronic, Inc.
Acknowledgements
We thank Colleen Gilbert, PharmD; HakanGürcan, MD; and Tim Peoples, MA, ELS, of Medtronic for their editorial assistance with preparing the manuscript and Ms. Madhawii for her secretarial assistance.
References
- Mauri L, Leon MB, Yeung AC, Negoita M, Keyes MJ, Massaro JM. Rationale and design of the clinical evaluation of the Resolute Zotarolimus-Eluting Coronary Stent System in the treatment of de novo lesions in native coronary arteries (the RESOLUTE US clinical trial). Am Heart J 2011;161:807-14.
- Joshi SR, Parikh RM. India - diabetes capital of the world: now heading towards hypertension. J Assoc Physicians India. 2007;55:323-4.
- Kaul U. Cardiovascular disease epidemic in India - a continuing problem. J Assoc Physicians India. 2012;60:9.
- Pimenta E, Calhoun DA. Resistant hypertension: incidence, prevalence, and prognosis. Circulation. 2012;125:1594-6.
- Gupta R, Guptha S. Strategies for initial management of hypertension. Indian J Med Res. 2010;132:531-42.
- Bertog SC, Sobotka PA, Sievert H. Renal denervation for hypertension. JACC Cardiovasc Interv. 2012;5:249-58.
- Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43:169-75.
- Sander GE, Giles TD. Resistant hypertension: concepts and approach to management. Curr Hypertens Rep. 2011;13:347-55.
- Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62:76-87.
- Mohan V, Deepa M, Farooq S, Datta M, Deepa R. Prevalence, awareness and control of hypertension in Chennai--The Chennai Urban Rural Epidemiology Study (CURES-52). J Assoc Physicians India. 2007;55:326-32.
- By Y, Mr NG, Ag U. Prevalence, awareness, treatment, and control of hypertension in rural areas of davanagere. Indian J Community Med. 2010;35:138-41.
- Burke GM, Sica DA, Frishman WH. Renal sympathetic denervation for the treatment of systemic hypertension. Cardiol Rev. 2012;20:274-8.
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Bohm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol. 2011;100:1049-57.
- Bigazzi R, Kogosov E, Campese VM. Altered norepinephrine turnover in the brain of rats with chronic renal failure. J Am Soc Nephrol.1994;4:1901-7.
- Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995;25:878-82.
- DiBona GF, Jones SY, Kopp UC. Renal mechanoreceptor dysfunction: an intermediate phenotype in spontaneously hypertensive rats. Hypertension. 1999;33:472-5.
- Kopp UC, Buckley-Bleiler RL. Impaired renorenal reflexes in two-kidney, one clip hypertensive rats. Hypertension. 1989;14:445-52.
- Kopp UC, Cicha MZ, Smith LA. Dietary sodium loading increases arterial pressure in afferent renal-denervated rats. Hypertension. 2003;42:968-73.
- Pan JY, Bishop VS, Ball NA, Haywood JR. Inability of dorsal spinal rhizotomy to prevent renal wrap hypertension in rats. Hypertension.1985;7:722-8.
- Ye S, Ozgur B, Campese VM. Renal afferent impulses, the posterior hypothalamus, and hypertension in rats with chronic renal failure. Kidney Int. 1997;51:722-7.
- Fujisawa Y, Nagai Y, Lei B, Nakano D, Fukui T, Hitomi H, Mori H, Masaki T, Nishiyama A. Roles of central renin-angiotensin system and afferent renal nerve in the control of systemic hemodynamics in rats. Hypertens Res. 2011;34:1228-32.
- Schlaich MP, Hering D, Sobotka PA, Krum H, Esler MD. Renal denervation in human hypertension: mechanisms, current findings, and future prospects. Curr Hypertens Rep. 2012;14:247-53.
- Onesti G, Kim KE, Greco JA, del Guercio ET, Fernandes M, Swartz C. Blood pressure regulation in end-stage renal disease and anephric man. Circ Res. 1975;36:145-52.
- Hampers CL, Skillman JJ, Lyons JH, Olsen JE, Merrill JP. A hemodynamic evaluation of bilateral nephrectomy and hemodialysis in hypertensive man. Circulation. 1967;35:272-88.
- Curtis JJ, Lucas BA, Kotchen TA, Luke RG. Surgical therapy for persistent hypertension after renal transplantation. Transplantation. 1981;31:125-8.
- Getts RT, Hazlett SM, Sharma SB, McGill RL, Biederman RW, Marcus RJ, Sandroni SE. Regression of left ventricular hypertrophy after bilateral nephrectomy. Nephrol Dial Transplant. 2006;21:1089-91.
- Devereux RB, Dahlöf B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110:1456-62.
- Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831-8.
- Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275-81.
- Krum H, Barman N, Schlaich M et al. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911-7.
- Schlaich MP. Long-term follow up of catheter-based renal denervation for resistant hypertension confirms durable blood pressure reduction Abstract presented at: Transcatheter Cardiovascular Therapeutics (TCT) Conference; 2012 22-26 October; Miami, FL, USA 2012.
- Esler M, Krum H, Schmieder R. Renal sympathetic denervation for treatment of resistant hypertension: two-year update from the Symplicity HTN-2 Randomized Controlled Trial. Abstract presented at: American College of Cardiology Conference; 2013 9-11 March; San Francisco, CA.
- Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 Randomized, Controlled Trial. Circulation. 2012;126:2976-2982.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903-9.
- Kaltenbach B, Franke J, Bertog SC, Steinberg DH, Hofmann I, Sievert H. Renal sympathetic denervation as second line therapy in mild resistant hypertension - a pilot study. Catheter Cardiovasc Interv 2013;81:335-9.
- Brandt MC, Reda S, Mahfoud F, Lenski M, Bohm M, Hoppe UC. Effects of renal sympathetic denervation on arterial stiffness and central hemodynamics in patients with resistant hypertension. J Am Coll Cardiol. 2012;60:1956-65.
- Mortensen K, Franzen K, Himmel F, Bode F, Schunkert H, Weil J, Reppel M. Catheter-based renal sympathetic denervation improves central hemodynamics and arterial stiffness: a pilot study. J Clin Hypertens (Greenwich). 2012;14:861-70.
- Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Böhm M. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012;60:419-24.
- Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA, Sobotka PA, Böhm M, Cremers B, Esler MD, Schlaich MP. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250-7.
- Dörr O, Liebetrau C, Möllmann H, Achenbach S, Sedding D, Szardien S, Willmer M, Rixe J, Troidl C, Elsässer A, Hamm C, Nef HM. Renal sympathetic denervation does not aggravate functional or structural renal damage. J Am Coll Cardiol. 2013;61:479-80.
- Himmel F, Weil J, Reppel M, Mortensen K, Franzen K, Ansgar L, Schunkert H, Bode F. Improved heart rate dynamics in patients undergoing percutaneous renal denervation. J Clin Hypertens Greenwich). 2012;14:654-5.
- Ukena C, Mahfoud F, Spies A, Kindermann I, Linz D, Cremers B, Laufs U, Neuberger HR, Böhm M. Effects of renal sympathetic denervation on heart rate and atrioventricular conduction in patients with resistant hypertension. Int J Cardiol. 2012;Aug 20. [Epub ahead of print]
- Ukena C, Mahfoud F, Kindermann I, Barth C, Lenski M, Kindermann M, Brandt MC, Hoppe UC, Krum H, Esler M, Sobotka PA, Böhm M. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol. 2011;58:1176-82.
- Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Böhm M, Hoppe UC. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. JACC. 2012;59:901-9.
- Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Böhm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940-6.
- Witkowski A, Prejbisz A, Florczak E, Kądziela J, Śliwiński P, Bieleń P, Michałowska I, Kabat M, Warchoł E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewicz A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559-65.
- Schlaich MP, Krum H, Sobotka PA, Esler MD. Renal denervation and hypertension. Am J Hypertens. 2011;24:635-42.
- Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol. 2011;100:1095-101.
- Steigerwald K, Titova A, Malle C, Kennerknecht E, Jilek C, Hausleiter J, Nährig JM, Laugwitz KL, Joner M. Morphological assessment of renal arteries after radiofrequency catheter-based sympathetic denervation in a porcine model. J Hypertens. 2012;30:2230-9.
- Böhm MS, M.P.; Krum, H.; Schmieder, R.E.; Mahfoud, F.; Sobotka, P.A.; Esler, M.D. 18 month pooled outcomes following renal sympathetic denervation In patients with resistant hypertension: from the Symplicity HTN-2 Trial. Poster session presented at: European Society of Cardiology Conference; 2012 25-29 August; Munich, Germany 2012.
- Cecka JM. Outcome statistics of renal transplants with an emphasis on long-term survival. Clin Transplant. 1994;8:324-7.
- Kaneku HK, Terasaki PI. Thirty year trend in kidney transplants: UCLA and UNOS Renal Transplant Registry. Clin Transplant. 2006;1:1-27.
- Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457-64.
- Voskuil M, Verloop WL, Blankestijn PJ, Agostoni P, Stella PR, Doevendans PA. Percutaneous renal denervation for the treatment of resistant essential hypertension; the first Dutch experience. Neth Heart J. 2011;19:319-23.
- Zuern CS, Rizas KD, Eick C, Stoleriu C, Bunk L, Barthel P, Balletshofer B, Gawaz M, Bauer A. Effects of renal sympathetic denervation on 24-hour blood pressure variability. Front Physiol. 2012;3:134.
- Fontenla A, García-Donaire JA, Hernández F, Segura J, Salgado R, Cerezo C, Ruilope LM, Arribas F. Management of resistant hypertension in a multidisciplinary unit of renal denervation: protocol and results. Rev Esp Cardiol. 2012;Dec 11. [Epub ahead of print]
- Lenski MM, F.; Barth, C.; Razouk, A.; Ukena, C.; Fischer, D.; Laufs, U.; Kindermann, I.; Boehm, M. Orthostatic function after renal sympathetic denervation in patients with resistant hypertension. Abstract presented at: European Society of Cardiology Conference; 2012 25-29 August; Munich, Germany 2012.
- Kline RL, Mercer PF. Functional reinnervation and development of supersensitivity to NE after renal denervation in rats. Am J Physiol. 1980;238:R353-8.
- Mogil RA, Itskovitz HD, Russell JH, Murphy JJ. Renal innervation and renin activity in salt metabolism and hypertension. Am J Physiol. 1969;216:693-7.
- Gazdar AF, Dammin GJ. Neural degeneration and regeneration in human renal transplants. N Engl J Med. 1970;283:222-4.
- Shannon JL, Headland R, MacIver AG, Ferryman SR, Barber PC, Howie AJ. Studies on the innervation of human renal allografts. J Pathol. 1998;186:109-15.
- Hansen JM AU, Fogh-Andersen N, Kanstrup IL, Bratholm P, Plum I, Strandgaard S. The transplanted human kidney does not achieve functional reinnervation. Clin Sci (Lond). 1994;87:13-20.
- Arrowood JA, Goudreau E, Minisi AJ, Davis AB, Mohanty PK. Evidence against reinnervation of cardiac vagal afferents after human orthotopic cardiac transplantation. Circulation. 1995;92:402-8.
- Ahmed H, Neuzil P, Skoda J, Petru J, Sediva L, Schejbalova M, Reddy VY. Renal sympathetic denervation using an irrigated radiofrequency ablation catheter for the management of drug-resistant hypertension. JACC Cardiovasc Interv. 2012;5:758-65.
- Ormiston JA, Watson T, van Pelt N, Stewart R, Haworth P, Stewart JT, Webster MW. First-in-human use of the OneShot renal denervation system from Covidien. EuroIntervention. 2013;8:1090-4.
- Prochnau D, Figulla HR, Surber R. Efficacy of renal denervation with a standard EP catheter in the 24-h ambulatory blood pressure monitoring-long-term follow-up. Int J Cardiol. 2012;157:447-8.
- Prochnau D LN, Kuehnert H, Figulla HR, Surber R. Catheter-based renal denervation for drug-resistant hypertension by using a standard electrophysiology catheter. EuroIntervention 2011.
- Mabin T, Sapoval M, Cabane V, Stemmett J, Iyer M. First experience with endovascular ultrasound renal denervation for the treatment of resistant hypertension. EuroIntervention. 2012;8:57-61.
- Prochnau D, Figulla HR, Surber R. Cryoenergy is effective in the treatment of resistant hypertension in non-responders to radiofrequency renal denervation. Int J Cardiol. 2012;Oct 22. [Epub ahead of print]
- Granada JF, Buszman PP. Renal denervation therapies for refractory hypertension. Curr Cardiol Rep. 2012;14:619-25.
- Aranda-Lara P, Martinez-Esteban MD, Munoz JJ, Hernandez-Marrero D. Renal sympathetic denervation: a new treatment strategy in the management of refractory arterial hypertension. Nefrologia 2012;32:555-7.Fox K, Ford I, Steg PG, Tendera M, Ferrari R. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–16.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528


