The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Original Article
Relationship of Arterial Stiffness with Hypertension and its Management in a North-Indian Population Free of Cardiovascular Disease
Volume 1, Jan 2012
Ravi R Kasliwal, Manish Bansal, Rahul Mehrotra, Naresh Trehan; Gurgaon, India
Page: 1-8
IntroductionArterial stiffness is being increasingly recognized as an important contributor of cardiovascular (CV) morbidity and mortality in many patient subsets, particularly in the elderly, hypertensives, and those with end-stage renal disease (1–10). Increased arterial stiffness results in rapid reflection of arterial pulse waveform from periphery which results in augmentation of central aortic systolic blood pressure (BP). At the same time, stiffening of aorta causes loss of elastic recoil which, coupled with absence of reflected wave during diastole, results in fall in central aortic diastolic pressure. While the increase in aortic systolic pressure increases cardiac afterload, the fall in aortic diastolic BP compromises coronary perfusion. Together, these pathological alterations adversely affect myocardial oxygen demand–supply relationship, thereby increasing CV morbidity and mortality (11).
Recent technical advancements have made noninvasive measurement of arterial stiffness feasible in clinical practice. Segmental pulse wave velocity (PWV), central aortic pressure, and augmentation index (AIx) are some of the measures of arterial stiffness which can be easily and reproducibly computed using the newer, noninvasive techniques (1). Several clinical trials have demonstrated utility of these parameters in CV risk stratification as well as in guiding antihypertensive therapy (1, 10, 12). Acknowledging the available evidence base, European Society of Cardiology has recommended assessment of arterial stiffness as an integral component of evaluation of any individual with hypertension (HT) (12).
Given the rapidly increasing burden of CV risk factors and CV disease in India, arterial stiffness measurement can be of great clinical value in Indians also. Unfortunately, the vast differences in CV disease epidemiology between Indians and the Western populations preclude direct extrapolation of results obtained in Western populations to Indians. Although a few trials have studied arterial stiffness in Indians also, the experience has been limited mainly to patients with known coronary artery disease (CAD) or diabetes mellitus (13–16). Since the greatest value of arterial stiffness assessment appears to be in hypertensive individuals, we sought this study to define the relationship of HT and its management with arterial stiffness in Indian subjects.
Methods
One hundred forty-four subjects who were free from any CV disease and who had presented to our out-patient department with one or more CV risk factors were included in the study. Absence of CV disease was confirmed by lack of symptoms suggestive of the same and a negative exercise stress test. Informed consent was obtained from all participants prior to their enrolment in the study.
Once enrolled, all subjects underwent clinical evaluation, biochemical investigations, and assessment of arterial stiffness. Clinical evaluation included detailed history regarding the presence or absence of CV risk factors, duration of CV risk factors, and use of antihypertensive medications, and general physical examination which included height, weight, and BP measurement and the examination of CV system. BP was measured in the right arm in supine position, using a standard sphygmomanometer. Biochemical investigations included fasting and 2-hour post-prandial blood glucose estimation and fasting lipid profile.
Arterial stiffness assessment (Fig. 1)
Assessment of arterial stiffness was performed using the PeriScope® device (Genesis Medical Systems Pvt Ltd, Hyderabad, India) which has been shown to have high degree of reproducibility for this purpose (17). This device is based on oscillometric method and records arterial pressure waveforms noninvasively. ECG-gated pressure waveforms are recorded simultaneously from both arms and both ankles. From these pressure waveforms, in-built software automatically calculates PWV for different vascular segments—brachial-ankle PWV (baPWV), carotid-femoral PWV (cfPWV), etc. Central aortic pressure and AIx are derived from brachial pressure waveforms, using a previously validated transfer function (1).
.jpg)
Assessment of arterial stiffness was performed in the morning, after 10 hours overnight fast. Participants were asked to refrain from smoking for at least 4 hours before the procedure. Ongoing medications were not discontinued but the morning dose was delayed until completion of the test. The procedure was performed in supine position. After 10 minutes of supine rest, four BP cuffs, which were connected to the PeriScope® device, were tied around both arms and both ankles. These BP cuffs carried oscillometric sensors to record pressure waveforms from the underlying arteries. ECG electrodes were applied on wrists and ankles to record ECG simultaneously. The machine then automatically inflated and deflated all the cuffs simultaneously while recording pressure waveforms from all the four sites. From these pressure waveforms, right and left baPWV, cfPWV, central aortic systolic and diastolic BP, and AIx were calculated by the system as mentioned above.
Although the absolute values of different arterial stiffness parameters were used for analysis, they were also compared with age- and gender-specific normal values already stored in the database of the system. This nomogram was based on the data derived from 988 healthy Indian subjects, free from major CV risk factors and CV disease (13).
Definition of CV risk factors
For the purpose of the present study, HT was defined according to Joint National Committee (JNC) 7 guidelines as systolic BP „d 140 mmHg or diastolic BP „d 90 mmHg or previous history of HT or self-reported use of antihypertensive medications (18). Diabetes mellitus was defined as fasting blood glucose > 126 mg/dl or 2-hour postprandial blood glucose > 200 mg/dl or pharmacological treatment for diabetes or previous history of diabetes mellitus. Dyslipidemia was defined as high total cholesterol (TC, >200 mg/dl) or low high density lipoprotein-cholesterol (HDL-C, <40 mg/dl in men and <50 mg/dl in women) or history of dyslipidemia. Family history was considered positive if a coronary event had occurred in a male first-degree relative before the age of 55 years or a female first-degree relative before the age of 65 years. Smoking or tobacco use in any form during the preceding month was also considered to be a CV risk factor.
The study was approved by an independent ethics committee.
Statistical Analysis
The data was managed on Microsoft excel spreadsheet (version 2007, Microsoft Corp, Seattle, Washington). Values were expressed as mean ± standard deviation or as percentages. Comparisons between the groups were carried out using Student’s unpaired t test, one-way analysis of variance, or chi-square test as appropriate. Correlations among different arterial stiffness measurements were assessed using Pearson’s correlation coefficients. A p value <0.05 was considered statistically significant. All statistical analyses were done using SPSS for Windows (release 15.0, SPSS Inc, Chicago, IL, USA).
Results
Clinical characteristics of the study population are described in Table 1. Mean age of the subjects was 45.7 years and three-forth were males.
Of all the subjects, 70.1% had HT, 15.3% had diabetes mellitus, 22.9% were current smokers, 75.7% had dyslipidemia, and 41.7% had family history of premature CAD. Majority (83%) were overweight (body-mass index >23.0 kg/m2) with overall mean body-mass index 27.3 kg/m2. More than half (55.0%) had high TC and 41.0% had low levels of HDL cholesterol.
Arterial stiffness parameters (Table 2)
Mean cfPWV was 935.5 ± 29.0 cm/sec in the overall study population. Mean right baPWV was 1354.6 ± 44.3 cm/sec and mean left baPWV was 1473.5 ± 326.4 cm/sec. When compared with the reference population, 57.6% had elevated cfPWV, 51.4% had elevated right baPWV, and 70.8% had elevated left baPWV.
Mean central aortic systolic BP was 115.1 ± 16.6 mmHg which was 18.5 mmHg lower than the brachial systolic BP (p <0.001). Mean central aortic diastolic BP was 79.3 ± 10.1 mmHg which was similar to brachial diastolic BP (79.4 ± 11.0, p >0.05). Average augmentation pressure was 6.9 mmHg with mean AIx 17.2 ± 11.6 mmHg.
Blood pressure control and relationship with arterial stiffness parameters
Of the 144 subjects, 101 were found to have HT. Fifty-four of the 101 hypertensive subjects (53.5%) had their BP controlled (systolic BP <140 mmHg and diastolic BP <90 mmHg). Of the remaining 47 subjects, 34 (72.3%) had BP in the range of stage 1 HT and 13 (27.7%) in the range of stage 2 HT as defined by the JNC 7 guidelines on HT (18). Among the patients with controlled BP, two-third were on antihypertensive medications, whereas one-third were on life style measures alone. Overall, 49 patients (48.5% of hypertensives) were on medications and 75.0% of them had their BP controlled below 140/90 mmHg level.
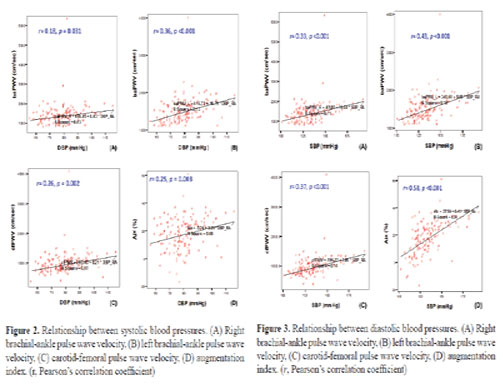
All arterial stiffness parameters (cfPWV, right and left baPWV and AIx) were significantly elevated in patients with HT (993.4 ± 382.6 cm/sec, 1430.7 ± 593.0 cm/sec, 1534.1 ± 347.4 cm/sec, and 20.0 ± 10.9%, respectively, in hypertensives and 799.5 ± 188.8 cm/sec, 1175.9 ± 277.7 cm/sec, 1331.1 ± 214.4 cm/sec, and 10.8 ± 10.7%, respectively, in non-hypertensives, p <0.01). Both, systolic and diastolic BP had significant correlation with cfPWV, right and left baPWV, and AIx (Figs. 2 and 3) with the strongest correlation obtained between systolic BP and AIx (Pearson’s correlation coefficient 0.58, p <0.001).

.jpg)
We divided all patients into four groups according to history of HT and level of BP control—group 0 (no history of HT and BP also normal, <140/90 mmHg), group 1 (history of HT but BP controlled), group 2 (no history of HT but elevated BP), and group 3 (history of HT and elevated BP). Although there was no significant difference in
systolic BP between group 0 and 1 (123.6 ± 8.5 mmHg and 126.4 ± 8.3 mmHg respectively, p=NS) and between group 2 and 3 (152.1 ± 16.7 mmHg and 152.1 ± 14.0 mmHg, respectively, p=NS), there was progressive increase in cfPWV, right baPWV, and left baPWV from group 0 to 3 (p <0.01, Fig. 4). This suggested that effective control of BP could result in improvement in arterial stiffness in known hypertensives. However, arterial stiffness in these individuals still remained elevated as compared to non-hypertensives with normal BP.
Relationship of arterial stiffness parameters with other CV risk factors (Table
There was no difference in cfPWV, right and left baPWV, and AIx in patients with or without diabetes mellitus, smoking, and family history of premature CAD and dyslipidemia (except increased right baPWV in patients without dyslipidemia; 1540.4 ± 880.5 vs 1288.0 ± 350.2 cm/sec, p = 0.02). In addition, there was no correlation between any of the above arterial stiffness parameters and fasting blood glucose, TC, and HDL-cholesterol
Discussios
Our study, which assessed arterial stiffness in North-Indian subjects who were free of CV disease, showed that (1) hypertensive patients have significantly increased arterial stiffness, (2) arterial stiffness increases progressively as BP increases, and (3) arterial stiffness can be improved with effective control of BP, though it still remains elevated as compared to non-hypertensives with normal BP.
HT is one of the most important determinants of arterial stiffness. A number of studies in Western populations have shown that arterial stiffness is significantly related to BP and is an independent predictor of CV events in hypertensives (1, 4–6, 10). Owing to its significant prognostic value, assessment of arterial stiffness has been recommended as a routine tool, wherever available, in the evaluation of hypertensive individuals (12). In addition, assessment of arterial stiffness may also be helpful in guiding antihypertensive therapy (1, 19). Various pharmacological and nonpharmacological interventions aimed at control of BP have been shown to improve arterial stiffness also, though not to the same extent (19–27). There is evidence to suggest that such improvement in arterial stiffness may be an important determinant of benefits achieved with antihypertensive therapy. In a study on patients with end-stage renal disease, Guerin et al. (28) found that lack of attenuation of arterial stiffness in spite of reduction in BP was an independent predictor of all-cause mortality. In addition, use of angiotensin-converting enzyme inhibitors was associated with better survival, an effect which was independent of BP reduction (28). In the Regression of Arterial Stiffness in a Controlled Double-Blind (REASON) trial, a combination of perindopril and indapamide produced much greater reduction in systolic BP and pulse pressure (both indirect measures of arterial stiffness) than atenolol, despite similar reduction in diastolic BP (29). The greater effect of perindopril/ indapamide combination on systolic BP and pulse pressure was associated with greater reduction in left ventricular mass also (30). The clinical value of such effects was evaluated further in the Conduit Artery Function Evaluation (CAFÉ) study (19), which was a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) (31). Despite a similar impact on brachial BP, amlodipine/perindopril based therapy resulted in substantially greater effect on central aortic pressure than atenolol-based regimen. Importantly, the greater effect of amlodipine/perindopril therapy on central aortic pressure was associated with reduced incidence of CV events and of renal impairment (19).
Several studies have measured arterial stiffness in Indian subjects also but none has adequately reported its relationship with HT. In a study on almost 4000 individuals, which included 988 healthy controls, Sridhar et al. found significantly elevated PWV in patients with diabetes, CAD, end-stage renal disease, or rheumatoid arthritis but effect of BP on PWV was not reported (13). Similarly, Kasliwal et al. measured baPWV in patients with and without CAD and found significantly elevated baPWV among those with CAD but its relationship with HT or BP was not studied (14). In the Chennai Rural Urban Epidemiologic Study, Mohan et al. studied 1985 subjects without known diabetes. Arterial stiffness, measured as AIx, increased with increasing risk of diabetes as estimated by Indian Diabetes Risk Score. An additional finding of the study was weak but statistically significant correlation between AIx and both systolic and diastolic BP (15). In another study, PWV in different arterial segments was found to correlate with systolic and diastolic BP but once again, no further information was available on its relationship with HT (16). In a study comparing 90 healthy South Asians living in Britain with 62 matched white Europeans, arterial stiffness was found to be significantly increased in South Asians. Interestingly, mean arterial pressure was found to be an independent predictor of arterial stiffness only in South Asians and not in white Europeans (32). Our study is therefore the first one to report effect of HT and BP control on arterial stiffness in Indian subjects
.jpg)
Limitations
The main limitation of our study was small sample size which precluded assessment of relative impact of different BP-lowering therapies (life style measures, drugs belonging to different classes) on arterial stiffness. Small sample size was also the likely reason responsible for lack of association between arterial stiffness and other
CV risk factors such as diabetes. In addition, we did not employ ambulatory BP monitoring to determine consistency of BP control. It is therefore difficult to determine whether relatively lower values of arterial stiffness parameters seen in hypertensive subjects with “controlled BP” (group 1) were merely reflective of lower BP values at the time of arterial stiffness assessment or if they actually reflected improvement in arterial stiffness. However, as mentioned above, it is noteworthy that the values of arterial stiffness parameters were much higher in hypertensive patients with “controlled BP” (group 1) than in non-hypertensive subjects with normal BP (group 0) in spite of almost similar average BP values in the two groups. Similarly, known hypertensives with elevated BP (group 3) had stiffer arteries than group 2 patients who had almost similar BP but were not previously known to be hypertensives. This suggests that BP at the time of arterial stiffness assessment alone was not the primary determinant of arterial stiffness and the lower values of arterial stiffness parameters seen in group 1 subjects were indeed result of improved arterial compliance.
Conclusions
Our study showed that in North-Indian adult subjects, free of CV disease, arterial stiffness is significantly increased among hypertensives and the degree of arterial stiffness increases progressively with increase in BP. More importantly, control of BP can bring about significant improvement in arterial stiffness as well. This makes assessment of arterial stiffness a potentially useful tool for tracking vasculoprotective effects of antihypertensive therapy in clinical practice. However, a larger study is needed to address prognostic implications of these findings and also to determine differential effect of various BP-lowering therapies on arterial stiffness in Indian subjects.
Acknowledgement
We sincerely thank Mr Arun Rawat, clinical research coordinator for the study, for his immense help in data collection and compilation.
Funding
No external source of funding.
Conflict of InterestNone
References
1. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006; 27:2588–605.
2. Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001; 21:2046–50.
3. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005; 111:3384–90.
4. Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002; 39:10–5.
5. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001; 37:1236–41.
6. Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003; 34:1203–6.
7. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999; 99:2434–9.
8. Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, Ishimura E, Tabata T, Nishizawa Y. Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol. 2001; 12:2117–24.
9. Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 1998; 32:570–4.
10. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010; 55:1318–27.
11. Milan A, Tosello F, Fabbri A, Vairo A, Leone D, Chiarlo M, Covella M, Veglio F.. Arterial stiffness: from physiology to clinical implications. High Blood Press Cardiovasc Prev. 2011; 18:1–12.
12. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty AM, Laurent S, Lindholm LH, Mancia G, Manolis A, Nilsson PM, Redon J, Schmieder RE, Struijker-Boudier HA, Viigimaa M, Filippatos G, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano JL. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007; 28:1462–536.
13. Sridhar Y, Naidu MUR, Usharani P, Raju YSN. Non-invasive evaluation of arterial stiffness in patients with increased risk of cardiovascular morbidity: A cross-sectional study. Indian J Pharmacol. 2007; 39:294–8.
14. Kasliwal RR, Bansal M, Bhargava K, Gupta H, Tandon S, Agrawal V. Carotid intima-media thickness and brachial-ankle pulse wave velocity in patients with and without coronary artery disease. Indian Heart J. 2004; 56:117–22.
15. Mohan V, Gokulakrishnan K, Ganesan A, Kumar SB. Association of Indian Diabetes Risk Score with arterial stiffness in Asian Indian nondiabetic subjects: the Chennai Urban Rural Epidemiology Study (CURES-84). J Diabetes Sci Technol. 2010; 4:337–43.
16. Kumaran K, Fall CH, Martyn CN, Vijayakumar M, Stein CE, Shier R. Left ventricular mass and arterial compliance: relation to coronary heart disease and its risk factors in South Indian adults. Int J Cardiol. 2002; 83:1–9.
17. Naidu MU, Reddy BM, Yashmaina S, Patnaik AN, Rani PU. Validity and reproducibility of arterial pulse wave velocity measurement using new device with oscillometric technique: a pilot study. Biomed Eng Online. 2005; 4:49.
18. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560–72.
19. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006; 113:1213–25.
20. Kool MJ, Lustermans FA, Breed JG, Struyker Boudier HA, Hoeks AP, Reneman RS, Van Bortel LM. The influence of perindopril and the diuretic combination amiloride + hydrochlorothiazide on the vessel wall properties of large arteries in hypertensive patients. J Hypertens. 1995; 13:839–48.
21. Mahmud A, Feely J. Reduction in arterial stiffness with angiotensin II antagonist is comparable with and additive to ACE inhibition. Am J Hypertens. 2002; 15:321–5.
22. White WB, Duprez D, St Hillaire R, Krause S, Roniker B, Kuse-Hamilton J, Weber MA. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension. 2003; 41:1021–6.
23. Maki-Petaja KM, Wilkinson IB. Anti-inflammatory drugs and statins for arterial stiffness reduction. Curr Pharm Des. 2009; 15:290–303.
24. Kingwell BA, Berry KL, Cameron JD, Jennings GL, Dart AM. Arterial compliance increases after moderate-intensity cycling. Am J Physiol. 1997; 273:H2186–91.
25. Balkestein EJ, van Aggel-Leijssen DP, van Baak MA, Struijker-Boudier HA, Van Bortel LM. The effect of weight loss with or without exercise training on large artery compliance in healthy obese men. J Hypertens. 1999; 17:1831–5.
26. Avolio AP, Clyde KM, Beard TC, Cooke HM, Ho KK, O'Rourke MF. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis. 1986; 6:166–9.
27. Sierksma A, Lebrun CE, van der Schouw YT, Grobbee DE, Lamberts SW, Hendriks HF, Bots ML. Alcohol consumption in relation to aortic stiffness and aortic wave reflections: a cross-sectional study in healthy postmenopausal women. Arterioscler Thromb Vasc Biol. 2004; 24:342–8.
28. Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001; 103:987–92.
29. Asmar RG, London GM, O'Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001; 38:922–6.
30. de Luca N, Asmar RG, London GM, O'Rourke MF, Safar ME. Selective reduction of cardiac mass and central blood pressure on low-dose combination perindopril/indapamide in hypertensive subjects. J Hypertens. 2004; 22:1623–30.
31. Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005; 366:895–906.
32. Gunarathne A, Patel JV, Gammon B, Hughes EA, Lip GY. Impact of mean arterial blood pressure on higher arterial stiffness indices in South Asians compared to white Europeans. J Hypertens. 2008; 26:1420–6.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528


