The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Books and Trials
Recent Landmark Trials
Volume 4, Jul 2015
Manish Bansal MD, DNB, Ravi R Kasliwal MD, DM, Gurgaon, India
J Clin Prev Cardiol 2015;4(3):78-80
Ezetimibe added to Statin Therapy after Acute Coronary SyndromesIMPROVE-IT: Improved Reduction of Outcomes: Vytorin Efficacy International Trial
Cannon CP, et al. N Engl J Med 2015; 372: 2387-97
Trial summary
This multicentric trial evaluated whether incremental reduction in LDL-C achieved with a non-statin drug when added to statin therapy could produce incremental CV risk reduction also.
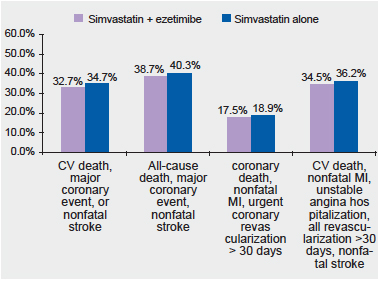
It was a double-blind, randomized placebo-controlled trial that included 18,144 patients presenting with an ACS within the preceding 10 days and having an LDL-C level of 50-100 mg/dL if already on lipid-lowering therapy or 50-125 mg/dL if not already on lipid-lowering therapy. The patients were randomized to receive a combination of simvastatin 40 mg/d and ezetimibe 10 mg/d or simvastatin 40 mg/d alone (with a matching placebo). The patients were followed up for a median period of 6 years. The primary end point was a composite of CV death, nonfatal myocardial infarction, unstable angina requiring re-hospitalization, coronary revascularization (>30 days after randomization), or nonfatal stroke.
The simvastatin-ezetimibe combination lowered LDL-C significantly more than simvastatin alone (average LDL-C 53.7 mg/dL versus 69.5 mg/dL, P<0.001). The combination therapy resulted in 6% lower risk of the primary end-point (32.7% versus 34.7%, P = 0.016) at the end of 7 years follow-up. No safety issues were observed with the combination treatment.
In conclusion, this trial showed that ezetimibe, when added to statin therapy, resulted in incremental lowering of LDL-C levels and incremental reduction in CV risk.
Perspective
Although statins are known to result in profound CV risk reduction in a wide variety of patient subsets, those treated with statin therapy still remain at considerably high residual risk of CV events. Numerous approaches, including more aggressive statin therapy and the use of several non-statin drugs, have been tried to reduce this residual CV risk in statin-treated patients.
Ezetimibe is an intestinal cholesterol absorption inhibitor and has a synergistic action with statins in lowering LDL-C. However, as statins are known to reduce CV risk not only by lowering LDL-C but also through their pleiotropic effects, it has always remained uncertain whether additional LDL-C reduction with ezetimibe could result in improvement in CV outcomes as well. The present trial seems to provide this answer by demonstrating increment reduction in CV risk when ezetimibe was added to simvastatin. However, several important points need to be considered when generalizing these findings. First, simvastatin 40 mg/d, which is a moderate intensity statin therapy, was used as the background statin therapy in this study (except in some patients initially). However, in current practice, high-intensity statin therapy, and not moderate-intensity statin therapy, is recommended for patients presenting with an acute coronary event. It is therefore not clear whether ezetimibe could have similarly reduced CV events even when added to high-intensity statin therapy. Second, in this study, although the incremental CV reduction with the combination therapy was statistically significant, the absolute effect (6% relative risk reduction over a period of 7 years) was modest at best. This raises question about real significance of ezetimibe mediated reduction in CV risk. On the contrary, the investigators argue that the patients included in this trial had relatively low LDL-C levels at baseline (mean value ~94 mg/dL) and it is possible that greater benefits from ezetimibe might have been seen if baseline LDL-C had been higher.
Follow-up of Glycemic Control and Cardiovascular Outcomes in Type 2 Diabetes
VADT: Veterans Affairs Diabetes Trial
Hayward RA, et al. N Engl J Med 2015;372:2197-206.
Trial summary
This was an extended follow-up of the previous VADT trial that had showed that intensive glucose lowering, as compared with standard therapy, did not significantly reduce the rate of major CV events at the end of a median follow-up of 5.6 years.
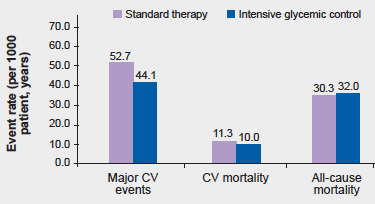
The trial had included 1791 military veterans who were randomly assigned to receive either intensive or standard glucose control for a period of 5.6 years. After the conclusion of the interventional phase, the participants were continued to be followed-up using central databases to record clinical outcomes. In addition, a majority of the participants also agreed to additional data collection by means of annual surveys and periodic chart reviews. Mean age of the study participants was ~60 years and more than 40% had already suffered a CV event at the time of inclusion in this study. The primary outcome was the time to the first major CV event (heart attack, stroke, new or worsening congestive heart failure, amputation for ischemic gangrene, or cardiovascular-related death).
During the initial intervention phase, the HbA1C levels were lower by 1.5 percentage points in the intensive-therapy group as compared to the standard-therapy group (median level, 6.9% vs. 8.4%) but this difference declined to 0.2 to 0.3 percentage points by 3 years after the trial ended. Over a median follow-up of 9.8 years, the intensive-therapy group resulted in 17% lower risk of the primary outcome than the standard therapy group (44.1 versus 52.7 events per 1000 patient-years, P = 0.04). However, there was no significant difference between the two groups in CV mortality or all-cause mortality.
Thus, in summary, this trial showed that intensive glucose control lowered CV risk over extended period of follow-up but did not improve the overall survival.
Perspective
Macrovascular complications are the major cause of morbidly and mortality in patients with type 2 diabetes. However, the effect of tight glycemic control on the risk of macrovascular complications remains unclear. Although the UKPDS (United Kingdom Prospective Diabetes Study) had showed that tight glycemic control could indeed prevent macrovascular events and improve overall survival, 3 other large trials [VADT, ADVANCE (the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trial, and the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial] showed no or minimal overall benefit of intensive glucose control on macrovascular outcomes.
In the present extended follow-up of VADT, intensive glycemic control was associated with a significant reduction in MACE but there was no survival benefit. The reduction in MACE was similar to that seen in follow-up studies of the UKPDS and the ACCORD trial. However, the effect on mortality had been variable in these trials with UKPDS showing reduced mortality, ACCORD showing increased mortality and VADT and ADVANCE showing no effect on mortality. These differences among trials could be related to the differences in the patient-characteristics (e.g. younger patients in UKPDS) and in the intensity of treatment regimen (e.g. UKPDS relatively less aggressive whereas ACCORD very intensive). The net conclusion that can be drawn from these studies is that intensive glycemic control can reduce the risk of macrovascular complications but this benefit may be offset by the increase in the mortality if glcyemic control is very aggressive, particularly in elderly patients.
Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease
PROMISE: Prospective Multicenter Imaging Study for Evaluation of Chest Pain
Douglas PS, et al. N Engl J Med 2015;372:1291-300.
Trial summary
The primary objective of this study was to determine whether, in patients presenting with susceptive CAD, initial anatomic testing with computed tomographic (CT) coronary angiography (CTCA) was superior to initial functional assessment with cardiac stress testing.
A total of 10,003 symptomatic patients (mean age ~61 years; majority having chest pain or dyspnea on exertion) were randomized to either CTCA or to functional testing (exercise electrocardiography, nuclear stress testing, or stress echocardiography) as the initial diagnostic strategy. The composite primary end point was death, myocardial infarction, hospitalization for unstable angina, or major procedural complication.
The mean pretest likelihood of obstructive CAD in the overall study cohort was 53.3±21.4%. Over a median follow-up period of 25 months, a primary end-point event occurred in 164 of 4996 patients in the CTCA group (3.3%) and in 151 of 5007 (3.0%) in the functional-testing group (P = 0.75). CTCA was associated with more frequent cardiac catheterizations (12.2% vs. 8.1% over 90 days) but fewer catheterizations showing no obstructive CAD than was functional testing (3.4% vs. 4.3%, P = 0.02). More patients in the CTCA group (6.2%) underwent revascularization within 90 days after randomization than in the functional-testing group (3.2%, P<0.001). Overall, initial strategy of CTCA was associated with greater radiation exposure, except in patients who underwent nuclear stress testing as the initial strategy.
Thus, this trial showed that in symptomatic patients with suspected CAD who required noninvasive testing, a strategy of initial CTCA did not improve 2-years clinical outcomes as compared with functional testing.
Perspective
In patients who present with symptoms suggestive of CAD (typically chest pain or dyspnea on exertion), the most important clinical goal is to determine the presence and the extent of obstructive CAD. However, the choice of initial diagnostic modality to achieve this objective remains unclear.
Traditionally, cardiac stress testing has been the mainstay of diagnostic evaluation in these patients and has a large evidence base to support its prognostic utility for this purpose. However, there are ongoing concerns about the overall diagnostic accuracy and the operator dependence of most of the cardiac stress tests. The recent introduction of CTCA has offered an alternate option with promises of much higher diagnostic accuracy and the ability to also detect non-obstructive yet prognostically important CAD. However, CTCA is associated with the problems of radiation exposure, additional cost, limited availability, and the risk of more frequent downstream testing because of detection of non-obstructive CAD. Moreover, as the presence of anatomic CAD is not synonymous with functionally significant CAD, the clinical superiority of anatomic testing with CTCA over functional testing is also not established. In this context, the present study has provided important insights. With a study design that resembles real-life clinical practice, the present study has shown that anatomic testing with CTCA is actually not superior to cardiac stress testing in improving clinical outcomes, at least over a period of two years, in patients with intermediate pretest likelihood of obstructive CAD.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528


