The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Books and Trials
Landmark Trials: Hypertension Management: What’s new!
Volume 2, Apr 2013
Rohit Mathur, MD, DM, Jodhpur, India
J Clin Prev Cardiol 2013;2(2):113-9
Hypertension has been a silent killer, the leading risk factor for myocardial infarction (MI), Heart failure, chronic renal failure and Stroke. It is responsible for the majority of office visits, number one reason for drug prescription and affects almost 25% of the world population. Prevalence of hypertension has been increasing steadily over the last decade. Recent studies point out that the prevalence of hypertension may be as high as 60% in the elderly population. With this high prevalence and high morbidity and mortality associated with it, hypertension has become one of the most common causes of death worldwide.
Looking at the burden of hypertension, we all understand the importance of adequate BP control. Here we review some important trials in the recent past which can change the practice of hypertension management significantly.
Effects of intensive blood-pressure control in type 2 diabetes mellitus
The ACCORD Study Group. N Engl J Med. 2010;362: 1575-85.
Trial Summary
Diabetes and hypertension are two common and deadly risk factors for cardiovascular mortality and morbidity. Their occurrence together in a patient can lead to complications more than two to three times more commonly than when only one of these is present. That is why JNC VII report had advised starting antihypertensivetreatment at a level of systolic blood pressure of 130 or more mmHg and also has made a target BP level at 130 mmHg or less in diabetic individuals. Therefore, it becomes interesting to study the effect of tighter BP control vis-à-vis routine BP control in diabetics.
ACCORD BP trial was a nonblinded trial in which 4733 participants (2362 to intensive blood pressure control and 2371 to standard therapy) with type 2 diabetes were randomly assigned to intensive therapy that targeted systolic blood pressures of less than 120 mmHg or standard therapy that targeted systolic blood pressures of less than 140 mmHg. Treatment strategies that are currently available in clinical practice were used to lower blood pressure. The primary composite outcome was nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. The mean follow-up was for 4.7 years.
After 1 year, the mean systolic blood pressure was 119.3 mmHg in the intensive therapy group and 133.5 mmHg in the standard-therapy group. The annual rate of the primary outcome was 1.87% in the intensive-therapy group and 2.09% in the standard-therapy group (hazard ratio with intensive therapy, 0.88; 95% confidence interval [CI], 0.73 to 1.06; p=0.20). The annual rates of death from any cause were 1.28% and 1.19% in the two groups, respectively (hazard ratio, 1.07; 95% CI, 0.85 to 1.35; p=0.55).
Serious adverse events attributed to antihypertensive treatment occurred in 77 of the 2362 participants in the intensive-therapy group (3.3%) and 30 of the 2371 participants in the standard-therapy group (1.3%) (p<0.001).
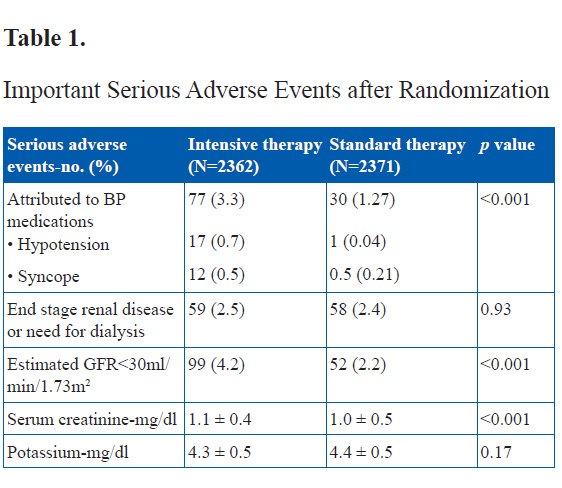

The two study groups did not differ significantly with respect to most of the other secondary outcomes. Nominally significant differences were seen in the rate of total stroke (0.32% per year in the intensive-therapy group vs. 0.53% per year in the standard-therapy group; hazard ratio, 0.59; 95% CI, 0.39 to 0.89; p=0.01) and in the rate of nonfatal stroke (0.30% per year in the intensive therapy group vs. 0.47% per year in the standard therapy group; hazard ratio, 0.63; 95% CI, 0.41 to 0.96; p=0.03).
The authors gave two reasons to explain the ACCORD results- First, It is possible that lowering systolic blood pressure from the mid-130s to approximately 120 mm Hg does not further reduce most cardiovascular events or the rate of death, and most of the benefit from lowering blood pressure is achieved by targeting a goal of less than 140 mmHg. Alternatively, it is possible that 5 years is not long enough to see significant cardiac benefits from the normalization of systolic blood pressure among persons with diabetes who have good glycemic control.
Perspective
BP reduction to optimum levels is always desirable, especially in diabetics. This trial however has shown that overzealous attempts to bring BP to far below recommended levels does not results in further lowering of clinical events over medium term follow up. Stricter BP reduction to <120 mmHg systolic BP resulted in no benefits in CV deaths, nonfatal stroke and nonfatal MI’s. However, a small benefit was seen in Stroke alone, in comparison to SBP <140 mmHg. This stricter BP reduction was associated with significantly increased side effects of hypotension and especially renal dysfunction. It will however be interesting to see the effect of tighter BP control in diabetics in the long term.
Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel-group trial
Morris J Brown, Gordon T McInnes, Cheraz Cherif Papst, Jack Zhang, Thomas M MacDonald. Lancet. 2011;377:312–20.
Adequate hypertension control more often than not requires more than one antihypertensive medication. Still the guidelines suggest starting treatment with a single class of agent. Usually another antihypertensive of different class is added sequentially to optimize BP control. In this study the authors tried to prove the hypothesis that starting treatment with combination antihypertensives of two mutually complimentary class will achieve target BP much earlier and with sustained effect. The mechanistic rationale for this hypothesis was that compensatory haemodynamic or neuroendocrine responses to individual drugs might attenuate their effectiveness, prevent catch up when a second drug is added, and contribute to adverse events that lead to the discontinuation of treatment.
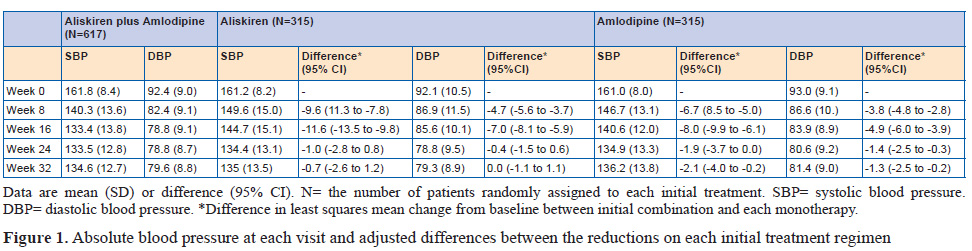
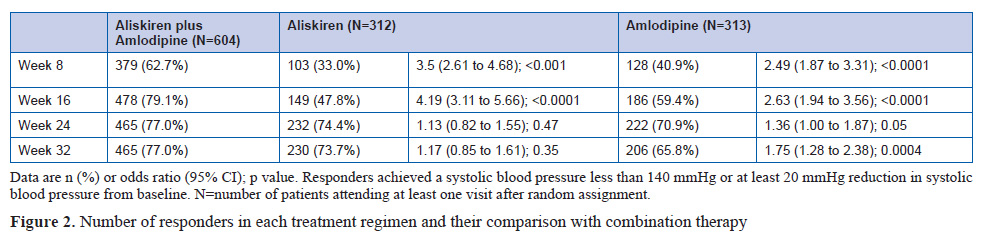
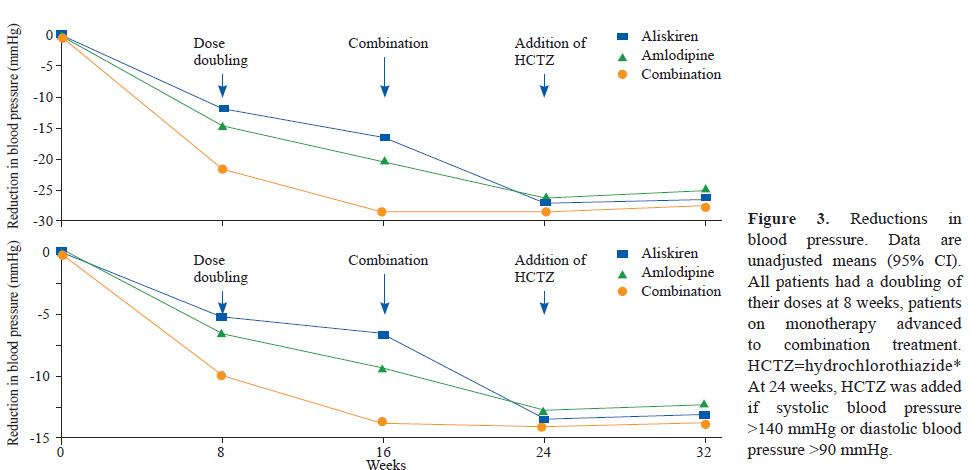
This was a double-blind, randomised, parallel-group, superiority trial including 318 patients assigned to aliskiren, 316 to amlodipine, and 620 to aliskiren plus amlodipine. 315 patients initially allocated to aliskiren, 315 allocated to amlodipine, and 617 allocated to aliskiren plus amlodipine were available for analysis. Eligible patients were randomly assigned at a ratio of 1:1:2 to treatment with 150 mg aliskiren plus placebo, 5 mg amlodipine plus placebo, or 150 mg aliskiren plus 5 mg amlodipine. At 8 weeks, the dose was doubled. After 16 weeks, all patients received the combination of 300 mg aliskiren plus 10 mg amlodipine. At week 24, patients received 12.5 mg hydrochlorothiazide or placebo if systolic blood pressure was greater than 140 mmHg or diastolic blood pressure was greater than 90 mmHg. The study ended at 32 weeks. The study had two sequential primary endpoints and hypotheses. The first, was the mean reduction from baseline of systolic blood pressure over weeks 8, 16, and 24, testing for superiority between the aliskiren plus amlodipine (initial combination) group and mean of each of the monotherapies. The second, tested only if the first hypothesis was positive, was the reduction from baseline in systolic blood pressure at week 24, a point in the study when all patients were in receipt of the same treatment, with the test of superiority favoring patients initially treated with the combination.
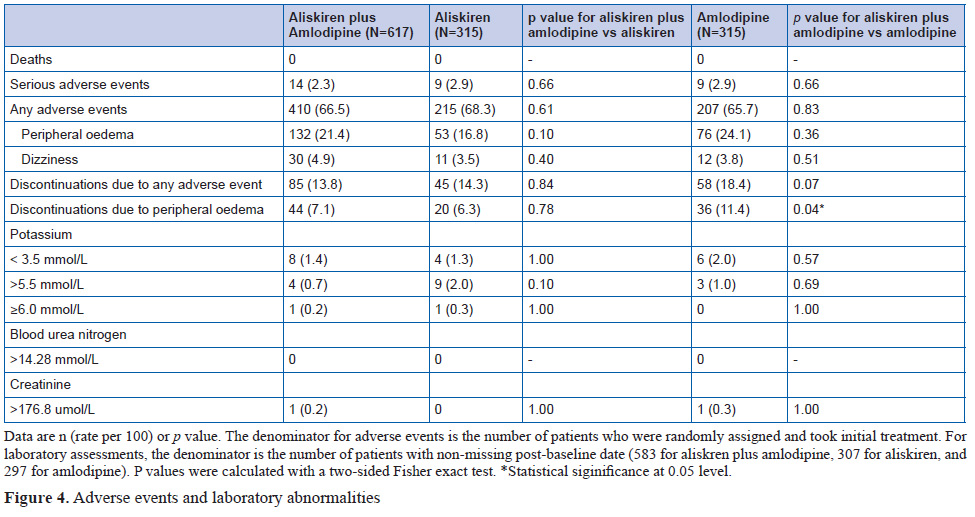
Patients given initial combination therapy had a 6.5 mm Hg (95% CI 5.3 to 7.7) greater reduction in mean systolic blood pressure than the monotherapy groups (p<0.0001). At 24 weeks,when all patients were on combination treatment, the difference was 1.4 mm Hg (95% CI –0.05 to 2.9; p=0.059).
Adverse events caused withdrawal of 85 patients (14%) from the initial aliskiren plus amlodipine group, 45 (14%) from the aliskiren group, and 58 (18%) from the amlodipine group. Adverse events were peripheral oedema, hypotension, or orthostatic hypotension.
Trial Summary
Adequate hypertension control more often than not requires more than one antihypertensive medication. Still the guidelines suggest starting treatment with a single class of agent. Usually another antihypertensive of different class is added sequentially to optimize BP control. In this study the authors tried to prove the hypothesis that starting treatment with combination antihypertensives of two mutually complimentary class will achieve target BP much earlier and with sustained effect. The mechanistic rationale for this hypothesis was that compensatory haemodynamic or neuroendocrine responses to individual drugs might attenuate their effectiveness, prevent catch up when a second drug is added, and contribute to adverse events that lead to the discontinuation of treatment.
This was a double-blind, randomised, parallel-group, superiority trial including 318 patients assigned to aliskiren, 316 to amlodipine, and 620 to aliskiren plus amlodipine. 315 patients initially allocated to aliskiren, 315 allocated to amlodipine, and 617 allocated to aliskiren plus amlodipine were available for analysis. Eligible patients were randomly assigned at a ratio of 1:1:2 to treatment with 150 mg aliskiren plus placebo, 5 mg amlodipine plus placebo, or 150 mg aliskiren plus 5 mg amlodipine. At 8 weeks, the dose was doubled. After 16 weeks, all patients received the combination of 300 mg aliskiren plus 10 mg amlodipine. At week 24, patients received 12.5 mg hydrochlorothiazide or placebo if systolic blood pressure was greater than 140 mmHg or diastolic blood pressure was greater than 90 mmHg. The study ended at 32 weeks. The study had two sequential primary endpoints and hypotheses. The first, was the mean reduction from baseline of systolic blood pressure over weeks 8, 16, and 24, testing for superiority between the aliskiren plus amlodipine (initial combination) group and mean of each of the monotherapies. The second, tested only if the first hypothesis was positive, was the reduction from baseline in systolic blood pressure at week 24, a point in the study when all patients were in receipt of the same treatment, with the test of superiority favoring patients initially treated with the combination.
Patients given initial combination therapy had a 6.5 mm Hg (95% CI 5.3 to 7.7) greater reduction in mean systolic blood pressure than the monotherapy groups (p<0.0001). At 24 weeks,when all patients were on combination treatment, the difference was 1.4 mm Hg (95% CI –0.05 to 2.9; p=0.059).
Adverse events caused withdrawal of 85 patients (14%) from the initial aliskiren plus amlodipine group, 45 (14%) from the aliskiren group, and 58 (18%) from the amlodipine group. Adverse events were peripheral oedema, hypotension, or orthostatic hypotension.




The findings show that patients randomly assigned to initial combination treatment with both aliskiren and amlodipine had substantially better mean blood pressure reduction over the first 24 weeks than did patients starting on either drug as monotherapy, with no cost in adverse events or withdrawals. Once the monotherapy patients progressed to combination therapy, their blood pressure fell towards, but never numerically caught up with, that of the initial combination group. Although the difference in systolic blood pressure between groups after 8 weeks on the combination regimen was less than the pre-trial hypothesis of 2.5 mmHg, 95% CIs suggest that a sustained difference of this order cannot be excluded.
Perspective
This trial shows importance of starting antihypertensive treatment with combination therapy instead of monotherapy and sequential addition of other antihypertensive drug. It was seen that combination of Aliskiren and Amlodipine resulted in far better BP control at week 8 and 16 weeks. Further, at 24th week when all the participants were on combination Aliskiren and Amlodipine, BP of those who were started on combination therapy was lower than in those started on monotherapy and given combination after 16th week. This signifies the benefit of early combination therapy to start with rather than addition later on.
Renal sympathetic denervation for treatment of drug-resistant hypertension one-year results from the Symplicity HTN-2 randomized, controlled trial
Murray D. Esler; Krum; Markus Schlaich; Roland E. Schmieder; Michael Bo¨hm; Paul A. Sobotka; for the Symplicity HTN-2 Investigators Circulation. 2012; 126:2976-2982
Trial Summary
Despite the availability of numerous effective antihypertensive medications, many hypertensive adults remain uncontrolled for various reasons, including inadequate treatment. According to the “Rule of Half” around 50 % of those taking antihypertensive medications can have suboptimal hypertension control. Patients who adhere to a prescribed pharmacological regimen of at least 3 drugs, including a diuretic are labeled as having uncontrolled or resistant hypertension. Estimates of resistant hypertension prevalence range from 13% to30% of adults receiving drug treatment for hypertension. These numbers reflect a serious health challenge given the observation that with every 20/10 mmHg increase in blood pressure, cardiovascular mortality doubles. Increased efferent sympathetic outflow to the kidneys causes elevation of blood pressure via release of renin, with subsequent activation of the renin- angiotensin- aldosterone system, increased tubular sodium retention, and reduced renal blood flow. Catheter-based renal denervation is a minimally invasive procedure involving the application of radiofrequency energy in short bursts along the length of the main renal arteries to ablate the renal nerves that lie within and just beyond the adventitia of the renal artery as they pass to the kidneys.
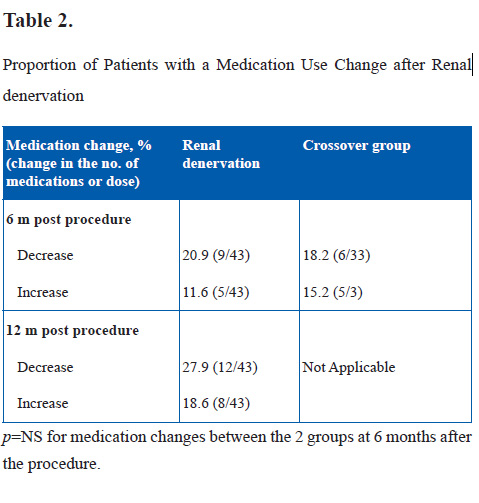
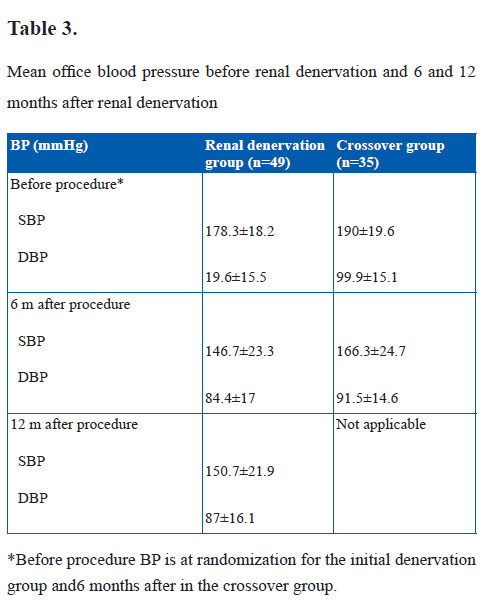
One hundred six patients were randomized to the renal denervation or control group after baseline screening for eligibility. Adult patients (aged 18–85 years) with essential hypertension, with a SBP ≥160 mmHg (≥150 mmHg if they had type 2 diabetes mellitus) were eligible for inclusion. Office baseline and follow-up blood pressure measurements were taken with an automated Omron HEM-705 monitor, and the average of 3 measurements was reported. After the 6-month primary end point was met, renal denervation in control patients was permitted. One-year results on patients randomized to immediate renal denervation (n=47) and 6-month postprocedure results for crossover patients are presented. At 12 months after the procedure, the mean fall in office systolic blood pressure in the initial renal denervation group (28.1 mmHg; 95% confidence interval, 35.4 to 20.7; p<0.001) was similar to the 6-month fall (31.7 mm Hg; 95% confidence interval, 38.3 to 25.0; p=0.16 versus 6-month change). The mean systolic blood pressure of the crossover group 6 months after the procedure was significantly lowered (from 190.0±19.6 to 166.3±24.7 mmHg; change, 23.7±27.5; p<0.001).
Before renal denervation treatment (6 months after randomization), the mean SBP of the crossover group had increased from 182.8±16.3 to 190.0±19.6 mmHg. This increase in blood pressure while patients are on a stable antihypertensive regimen may represent a natural tendency for blood pressure to increase in patients with treatment-resistant hypertension and suggests a potential cost in delaying renal denervation treatment.
There are certain shortcomings of the study. 24-hour blood pressure monitoring was lacking. It is possible, although not definitively established, that with renal denervation, reactive elements in blood pressure, such as might operate with office blood pressure measurements, are lowered more than the less reactive components that manifest in 24-hour blood pressure monitoring. Additionally, there was no blinding of patients or staff measuring the blood pressure response to the renal denervation intervention.
In the crossover group, there was 1 renal artery dissection during guide catheter insertion, before denervation, corrected by renal artery stenting, and 1 hypotensive episode, which resolved with medication adjustment.
All in all, this trial suggests that renal artery denervation with the Symplicity system is quite effective and at the same time very safe tool for the control of resistant hypertension.


Perspective
Surgical sympathetic denervation was an effective treatment for hypertension in older time, but with invention of effective anti-hypertensive medications and the associated morbidity of extensive surgical sympathetic denervation this procedure was forgotten. With the increasing prevalence of resistant hypertension and increasing zeal to treat resistant cases, sympathetic denervation has again come in to light. This trial shows very efficiently the safety, tolerability and good efficacy of Catheter based renal sympathetic denervation procedure. Patients treated with catheter based renal denervation had 28 mmHg fall in mean BP, with only 1 patient having major complication.
Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin–angiotensin–aldosterone system inhibitors involving 158,998 patients
Laura C. van Vark*, Michel Bertrand, K. Martijn Akkerhuis, Jasper J. Brugts, Kim Fox, Jean-Jacques Mourad, and Eric Boersma. Eur Heart J. 2012;33:2088-97.
Trial Summary
Till now, mortality benefits of any anti-hypertensive medication are considered to be due to its blood pressure reducing effect only. It is not known whether any antihypertensive can have mortality benefits over and above its blood pressure reducing effect.
In this meta-analysis, a pooled analysis of 20 cardiovascular mortality and morbidity trials was performed. The authors compared RAAS blockade (ACE inhibitors and ARBs) with contemporary anti-hypertensive medications. Patients with heart failure, ACS, acute stroke, atrial fibrillation, post cardiac surgery or on hemodialysis were excluded because of proven benefit of RAAS blockade in these groups.
The end point of the analysis was all cause mortality or CV mortality in the long term. In total of 158,998 patients were randomized to RAAS inhibitor therapy or control treatment (71,401 to RAAS inhibitors; 87,597 to control). ACE inhibitors were used as the active treatment in seven trials while thirteen trials, of which five were placebo-controlled, allocated participants to an ARB as the active treatment.
Mean follow-up was of 4.3 years. The incidence of all cause mortality was 20.9 deaths per 1000 patient-years in RAAS group while the same was 23.3 deaths per 1000 patient-years in control group. In all 20 trials grouped together, treatment with an RAAS inhibition was associated with a statistically significant 5% reduction in all-cause mortality (HR: 0.95, 95% CI: 0.91–1.00, p=0.032). Similarly there was a significant 7% overall reduction in cardiovascular mortality (HR: 0.93, 95% CI: 0.88–0.99, p=0.018) with RAAS blockade in comparison with contemporary medication. Almost all the benefit of RAAS blockade was driven by the benefit of ACE inhibitors only. ACE inhibitors were associated with a statistically significant 10% reduction in all-cause mortality. No significant mortality reduction could be demonstrated with ARB treatment. This difference in the treatment effect between ACE inhibitors and ARBs was statistically significant (p-value for interaction 0.036). The mortality reduction was largest in trials with the highest mean baseline BP values and larger BP reduction after antihypertensive medication.
The authors postulate that difference in mechanism of action of ACE inhibitors and ARBs (ACE inhibitors are characterized by a decrease in the degradation of bradykinin leading to a release of nitric oxide and prostaglandins resulting in additional vasodilatation) and BP independent CAD mortality benefit seen with ACE inhibitors but not with ARBs account for the difference seen in hypertensives as far as all cause and CV mortality are considered. However, as it was a meta-analysis of all available studies, information on background therapy and co morbidities was not available.
The authors postulate that difference in mechanism of action of ACE inhibitors and ARBs (ACE inhibitors are characterized by a decrease in the degradation of bradykinin leading to a release of nitric oxide and prostaglandins resulting in additional vasodilatation) and BP independent CAD mortality benefit seen with ACE inhibitors but not with ARBs account for the difference seen in hypertensives as far as all cause and CV mortality are considered. However, as it was a meta-analysis of all available studies, information on background therapy and co morbidities was not available.
Perspective
This meta-analysis throws light on beneficial effect of RAAS blockade especially ACE inhibitors in patients with hypertension without other co morbidity. It has shown that use of RAAS blockade resulted in significant reduction in all cause and CV mortality. Almost all the benefit of RAAS blockade was seen with ACE inhibitors only and the difference between ACE Inhibitors and ARB’s was significant. The implications of this meta-analysis can be important as till now the guidelines do not give preference to one RAAS blockade over other.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528


