The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Original Article
Echocardiographic Evaluation of Left Ventricular Diastolic Function in Healthy Neonates
Volume 1, Oct 2012
S R Mittal, MD, DM, Ajmer, Rajasthan, India.
J Clin Prev Cardiol 2012;1(4):161-6
There is increasing awareness of importance of diastolic dysfunction in the evaluation of neonates with congenital heart disease. There is, however, scanty literature regarding echocardiographic evaluation of left ventricular diastolic functions in healthy neonates. Most of the previous studies in children have either not studied neonates (1–7) or combined them with infants or older children (8,9). Studies which have been performed with an aim to evaluate healthy neonates have focused on systolic function or mitral valve flow pattern (10,11). Many variables now considered essential for the evaluation of diastolic function have not been studied. There is no literature regarding left atrial dimension, pulmonary vein flow pattern, velocity of propagation of mitral flow, ratio between early diastolic mitral flow and early diastolic velocity of tissue Doppler imaging (TDI) (E/Ea ratio), isovolumic contraction time (IVCT), isovolumic relaxation time (IVRT) and myocardial performance index (MPI) in healthy neonates. We performed detailed echocardiographic evaluation of left ventricular diastolic functions in healthy neonates.Material and Method
The study was approved by institutional ethical committee. Informed consent was obtained from all parents. Eighteen healthy neonates were selected out of neonates referred for echocardiography. They had no cardiorespiratory or systemic disease. They were not receiving any drug and were breathing room air. No subject had any abnormality on two-dimensional or Doppler echocardiography. Twelve neonates were male and six were females. Mean age was 4.50±2.50 days. Mean heart rate was 120.67±1.34/min. Mean weight was 2.77±0.60 kg.
Exclusion criteria
Neonates with history of asphyxia were excluded as even mild asphyxia is known to affect left ventricular functions (12). Neonates with patent ductus arteriosus with left to right shunt were excluded as such shunts could affect left ventricular diastolic function (13). Neonates with any atrial septal defect with left to right shunt or those with any evidence of pulmonary artery hypertension were also excluded because right ventricular volume and pressure overload may affect left ventricular functions (14). Neonates with any valvular lesion other than isolated trivial tricuspid regurgitation (eight neonates) were also excluded. Isolated trivial tricuspid insufficiency does not affect left ventricular function evaluation (7). Those with any rhythm disorder or conduction defect were also excluded. Neonates of diabetic mothers were also excluded as such infants are known to have left ventricular dysfunction (15). Preterm neonates, those with intrauterine growth retardation and those with chromosomal abnormalities/syndromes were also excluded as such neonates are likely to have cardiac function abnormalities (16,17).
Echocardiography
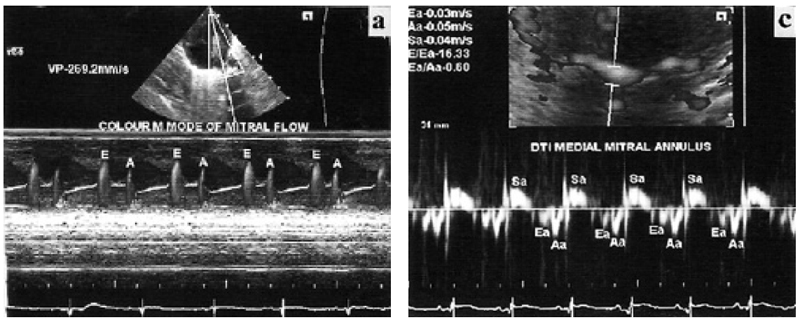
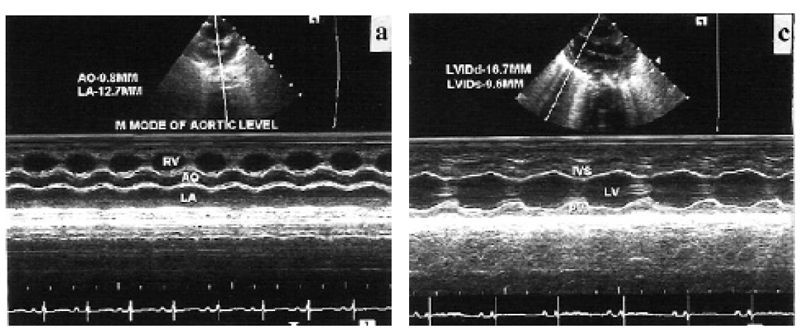
It was performed on Siemens Acuson X 300 with facility for TDI. Phased array transducer with frequency of 4–8 MHz was used. Chloral hydrate was used for sedation when required. All examinations were performed in slight right anterior oblique position. Left ventricular and left atrial dimensions and anterior mitral leaflet motion were recorded in left parasternal long axis view (Fig. 1a¬–c) as per standard guidelines in adults (18). To record mitral flow velocities with the pulsed Doppler method, sample volume was placed at the level of tip of both leaflets during diastole. Early (E) and late diastolic velocities were recorded (Fig. 1d). Duration and velocity time integral (VTI) were analyzed and E/A ratio was calculated. Mitral flow propagation velocities (Vp) were recorded in the same view using color Doppler and keeping cursor in mitral flow. Slopes of early and late diastolic flow were measured (Fig. 2a). Pulmonary vein flow Doppler was recorded from apical four-chamber view by keeping sample volume inside the right upper pulmonary vein, which is parallel to the ultrasound beam in this view. Systolic, diastolic and atrial reversal waves were recorded (Fig. 2b). Velocity, VTI and duration were analyzed. Difference between mitral flow A-wave duration and pulmonary vein atrial reversal duration (A-Ar) was calculated. TDI was performed in apical four -chamber view (19). Sample volume was kept over medial and lateral mitral annulus. Ultrasound beam was kept perpendicular to the plane of the annulus to minimize angle of incidence. Systolic velocity (Sa), early diastolic velocity (Ea), late diastolic velocity (Aa) were recorded (Fig. 2c,d). Their VTI, IVCT and IVRT and ejection time (ET) were analyzed and MPI was calculated. Due to fast heart rate and respiratory rate, effect of single respiratory cycle is much less in neonates (7). Furthermore any phase lag between respiration and ventricular filling is difficult to discern at fast heart and respiratory rates (7). Therefore, average of consecutive five cardiac cycles was taken for each parameter to minimize the effect of respiration.

.jpg)


Data are presented as mean±SD.
Results
M-mode parameters are shows in Table 1. Interventricular septum and left ventricular posterior wall had good systolic thickening. Left ventricular ejection fraction and fractional shortening were normal as compared to adults. Mitral valve diastolic movement showed normal pattern as seen in adults. Pulsed Doppler parameters of mitral flow are shown in Table 2. E-wave velocity was more than A-wave velocity. E-wave de-acceleration time was within normal range as seen in adults. Velocity of propagation of mitral flow are shown in Table 3. Velocity of early diastolic flow was more than late diastolic flow. Pulsed Doppler parameters of pulmonary vein flow are shown in Table 4. Systolic and diastolic flow velocities were nearly equal. VTI of systolic wave was more than diastolic wave. Duration of mitral flow A-wave was more than duration of pulmonary vein flow atrial reversal wave. Doppler tissue imaging velocities of medial and lateral mitral annulus are shows in Table 5. Late diastolic wave (Aa) velocity and VTI were more than velocity and VTI of early diastolic wave (Ea).
.jpg)
IVS, interventricular septum; PW, posterior wall; ED, end diastolic; ES, end systolic; LV, left ventricular cavity; EDD, end diastolic dimension; ESD, end systolic dimension; EF, ejection fraction; FS, fractional shortening; LA, anteroposterior dimension of left atrium; E, early diastolic opening of mitral valve; A, late diastolic opening of mitral valve.
.jpg)
E, early diastolic flow; PV, peak velocity; VTI, velocity time integral; DT, de-acceleration time; A, late diastolic flow.
.jpg)
.jpg)
VTI, velocity time integral; DT, de-acceleration time; A-AR, difference between mitral flow A-wave duration and pulmonary vein atrial reversal (AR) duration.
.jpg)
Ea, early diastolic velocity; VTI, velocity time integral; Aa, late diastolic velocity; Sa, systolic velocity; E, early diastolic velocity of mitral Doppler flow; IVCT, isovolumic contraction time; IVRT, isovolumic relaxation time; MPI, myocardial performance index.
Discussion
Left ventricular dimensions, ejection fraction and fractional shortening were similar to observations of previous workers (10). M-mode evaluation of diastolic movement of mitral valve and EF slope were normal. There is no mention of these findings in healthy neonates in previous literature. Previous workers have also observed that E-wave velocity is higher than A-wave velocity in Doppler evaluation of mitral flow (6,10). There is, however, no literature regarding de-acceleration time of E-wave and duration of A-wave. We observed that E-wave de-acceleration time was normal as compared to adults. A-wave duration was more than the duration of atrial reversal wave of pulmonary vein flow, as seen in normal adults. Mitral flow pulsed Doppler parameters had a pattern similar to normal adults. Velocity of propagation (Vp) of mitral flow was lower than that of adults. It was in the range considered suggestive of diastolic dysfunction in adults.
In pulmonary vein flow, diastolic velocity was nearly similar to systolic flow velocity. This finding is also suggestive of diastolic dysfunction in normal adults. On Doppler tissue imaging of medial and lateral mitral annulus, velocity and VTI of Aa-wave were more than Ea velocity. This finding is also considered suggestive of noncompliant pattern in adults. Thus, Vp, pulmonary vein flow pattern and TDI suggested noncomplaint pattern in healthy neonates.
Aa velocity greater than Ea velocity is observed in full-term fetuses (20,21). Sequential TDI of same fetuses has shown progressive increase in Ea velocity without change in Aa velocity (21). Pulsed Doppler study of diastolic filling patterns in fetuses has shown that E-wave velocity increases mainly after 25 weeks of gestation (16). Mori et al. (10) have observed progressive increase in peak E-wave velocity of mitral Doppler flow with number of hours after birth. Sequential studies of mitral flow in neonates have shown an increase in ventricular relaxation, at 1 month as compared to 6 days of neonatal life (6) and on day 2 as compared to day 1 (7). Studies in relatively older children have shown that early diastolic mitral flow velocity increases with growth, most significantly over the first year of life (8).
These observations suggest that there are age-related maturational changes in diastolic function (5). Neonatal period is a transition zone where diastolic function evaluation still shows a less compliant pattern. Experimental studies have also observed less complaint state in newborn (22). This could be due to relatively high content of total collagen and type 1 collagen in neonatal heart (23).
Our observations and review of literature suggest that a less complaint pattern is normal in neonates. Only significant deviation from observed values should be suggestive of diastolic dysfunction in neonates. We observed that mitral valve EF slope and mitral flow pattern were normal as compared to adults. Although these parameters are load-dependent, their abnormality may suggest diastolic dysfunction in neonates. Further studies in neonates with cardiac diseases known to affect diastolic function will be more informative.
In pulmonary vein flow, diastolic velocity was nearly similar to systolic flow velocity. This finding is also suggestive of diastolic dysfunction in normal adults. On Doppler tissue imaging of medial and lateral mitral annulus, velocity and VTI of Aa-wave were more than Ea velocity. This finding is also considered suggestive of noncompliant pattern in adults. Thus, Vp, pulmonary vein flow pattern and TDI suggested noncomplaint pattern in healthy neonates.
Aa velocity greater than Ea velocity is observed in full-term fetuses (20,21). Sequential TDI of same fetuses has shown progressive increase in Ea velocity without change in Aa velocity (21). Pulsed Doppler study of diastolic filling patterns in fetuses has shown that E-wave velocity increases mainly after 25 weeks of gestation (16). Mori et al. (10) have observed progressive increase in peak E-wave velocity of mitral Doppler flow with number of hours after birth. Sequential studies of mitral flow in neonates have shown an increase in ventricular relaxation, at 1 month as compared to 6 days of neonatal life (6) and on day 2 as compared to day 1 (7). Studies in relatively older children have shown that early diastolic mitral flow velocity increases with growth, most significantly over the first year of life (8).
These observations suggest that there are age-related maturational changes in diastolic function (5). Neonatal period is a transition zone where diastolic function evaluation still shows a less compliant pattern. Experimental studies have also observed less complaint state in newborn (22). This could be due to relatively high content of total collagen and type 1 collagen in neonatal heart (23).
Our observations and review of literature suggest that a less complaint pattern is normal in neonates. Only significant deviation from observed values should be suggestive of diastolic dysfunction in neonates. We observed that mitral valve EF slope and mitral flow pattern were normal as compared to adults. Although these parameters are load-dependent, their abnormality may suggest diastolic dysfunction in neonates. Further studies in neonates with cardiac diseases known to affect diastolic function will be more informative.
Limitations
In this study, small number of subjects was a limitation. This was due to the strict exclusion criteria to select truly healthy neonates out of those referred to the department for echocardiography.
Grant Support
None
Source of Funding
None
Conflict of Interest
None
References
References
- Kapusta L, Thijssen JM, Cuypers MHM, Peer PGM, Daniels O. Assessment of myocardial velocities in healthy children using tissue Doppler imaging. Ultrasound Med Biol. 2000; 26:229–37.
- O’Leary PW, Durongpisitkul K, Cordes TM, Bailey KR, Hagler DJ, Tajik J, Seward JB. Diastolic ventricular function in children: a Doppler echocardiographic study establishing normal values and predictors of increased ventricular end-diastolic pressure. Mayo Clin Proc. 1998; 73:616–28.
- Rychik J, Tian JY. Quantitative assessment of myocardial tissue velocities in normal children with Doppler tissue imaging. Am J Cardiol. 1996; 77:1254–7.
- Mori K, Hayabuchi Y, Kuroda Y, Nii M, Manabe T. Left ventricular wall motion velocities in healthy children measured by pulsed wave Doppler tissue echocardiography: normal values and relation to age and heart rate. J Am Soc Echocardiogr. 2000; 13:1002–11.
- Bu’ Lock FA, Mott MG, Martin RP. Left ventricular diastolic function in children measured by Doppler echocardiography: normal values and relation with growth. Br Heart J. 1995; 73:334–9.
- Ichihashi K, Ewert P, Welmitz G, Lange PE. Changes in cardiac diastolic function in neonates. Heart Vessel. 1997; 12:21–620.
- Riggs TW, Rodriguez R, Snider AR, Batton D. Doppler echocardiographic evaluation of right and left ventricular diastolic function in normal neonates. J Am Coll Cardiol. 1989; 13:700–5.
- Eidem BW, Mc Mahon CJ, Cohen RR, Wu J, Finkelshteyn I, Kovalchin JP, Ayres NA, Bezold LI, O’Brian Smith E, Pignatelli RH. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004; 17:212–21.
- Harada K, Orino T, Yasuoka K, Tamura M, Takada G. Tissue Doppler imaging of left and right ventricles in normal children. Tohoku J Exp Med. 2000; 191:21–9.
- Mori K, Nakagawa R, Nii M, Edagawa T, Takehara Y, Inoue M, Kuroda Y. Pulsed wave Doppler tissue echocardiography assessment of the long axis function of the right and left ventricles during the early neonatal period. Heart. 2004; 90:175–80.
- Takenaka K, Dabestani A, Waffarn F, Gardin JM, Henry WL. Effect of left ventricular size on early diastolic left ventricular filling in neonates and in adults. Am J Cardiol. 1987; 59:138–41.
- Ichihashi K, Yada Y, Takahashi N, Honma Y, Momoni M. Utility of Doppler derived index combining systolic & diastolic performance (Tei index) for detecting hypoxia cardiac damage in newborn. J Perinat Med. 2005; 33:542–52.
- Schmitz L, Stiller B, Koch H, Koehne P, Lange P. Diastolic left ventricular function in pre term infants with a patent ductus arteriosus: a serial Doppler echocardiographic study. Early Hum Dev. 2004; 76:91–100.
- Kim WH , Otsuji Y, Seward JB, Tei C. Estimation of left ventricular function in right ventricular volume and pressure overload. Detection of early left ventricular dysfunction by Tei-index. Jpn Heart J. 1999; 40:145–54.
- Tsutsumi T, Ishil M, Eto G, Hota M, Kato H. Serial evaluation for myocardial performance in fetuses and neonates using a new Doppler index. Pediatr Int. 1999; 41:722–7.
- Harda K, Rice MJ, Shiato T, Ishii M, McDonald RW, Reller MD, Sahn DJ. Gestational age and growth related alterations in fetal right and left ventricular diastolic filling patterns. Am J Cardiol. 1997; 79:173–7.
- Rizzo G, Arduini D. Fetal cardiac functions in intrauterine growth retardation. Am J Obstet Gynecol. 1991; 165:876–82.
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for the quantification of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989; 2:358–67.
- Wei Y, Xu JK, Xu T, Fan J, Tan SY. Left ventricular systolic function of newborns with asphyxia evaluated by tissue Doppler imaging. Pediatr Cardiol. 2009; 30:741–6.
- Paladini D, Lambertia A, Teodora A, Arienzo M, Tartaglione A, Martinelli P. Tissue Doppler imaging of the fetal heart. Ultrasound Obstet Gynecol. 2000; 16:530–5.
- Harada K, Tsuda A, Orino T, Tanaka T, Takada G. Tissue Doppler imaging in the normal fetus. Int J Cardiol. 1999; 71:227–34.
- Ramero T, Covell J, Friedman WF. A comparison of pressure volume relations of the fetal, newborn and adult heart. Am J Physiol. 1972; 49:1285–90.
- Marizianowski MMH, Van Der Loos CM, Mohrschladt MF, Beeker AE. The neonatal heart has a relatively high content of total collagen and type 1 collagen: a condition that may explain the less compliant state. J Am Coll Cardiol. 1994; 23:1204–8.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528