The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Review Article
Cardiovascular Risk Stratification In Indians
Volume 4, Jan 2015
Manish Bansal, MD, DNB Cardiology; Rahul Mehrotra, MD, DNB Cardiology; Ravi R Kasliwal, MD, DM, Gurgaon, India
J Clin Prev Cardiol. 2015;4(1):7-17
Estimation of the risk of occurrence of future atherosclerotic cardiovascular (CV) events is an important step in the management of the patients requiring primary prevention of CV disease. The ability to quantify future CV risk allows objective assessment of the ‘seriousness’ of the illness, provides a means to communicate the same to the patient and his family, and most importantly, forms the basis on which a number of important therapeutic decisions are taken. The aggressiveness of the lifestyle changes, the need and intensity of statin therapy, use of aspirin, etc. are some of those decisions that are largely determined by the perceived future risk of vascular disease in a given individual. It is therefore important to be aware of the available risk assessment tools, the advantages and the limitations of different risk assessment methods available and their relevance to the patient population in question. Indians are known to a represent a unique population as far as CV disease is concerned. As such, there is a need to have separate risk assessment approach in Indian patients.Clinical Risk Algorithms
Traditionally, assessment of CV risk in any given individual is performed by determining the presence and severity of major CV risk factors and subsequently using risk algorithms and prediction charts to determine the 10-year CV risk. Ten-year risk <10% is generally considered to indicate low risk, 10-20% intermediate risk and >20% indicates high risk. However, different guidelines have used different thresholds for defining high-risk. Most have indicated that >15-20% risk of occurrence of vascular events [death from CV causes, myocardial infarction (MI), or stroke] over 10 years should be considered as ndicative of high risk.
A number of risk assessment tools are available for this purpose, such as, the Framingham risk score (FRS) (1,2), Prospective Cardiovascular Munster Score (PROCAM) (3), World Health ganization/International Society of Hypertension (WHO/ISH) CVD risk prediction charts (4), Systemic Coronary Risk Evaluation (SCORE)(5), QRISK (6-8), Reynolds score (9,10), New Zealand score (11), American College of Cardiology/ American Heart Association (ACC/AHA) pooled cohort equations (12), the 3rd Joint British Societies’ (JBS3) risk calculator (13), etc. Among them, FRS is the oldest and the most widely used risk assessment algorithm in clinical practice.
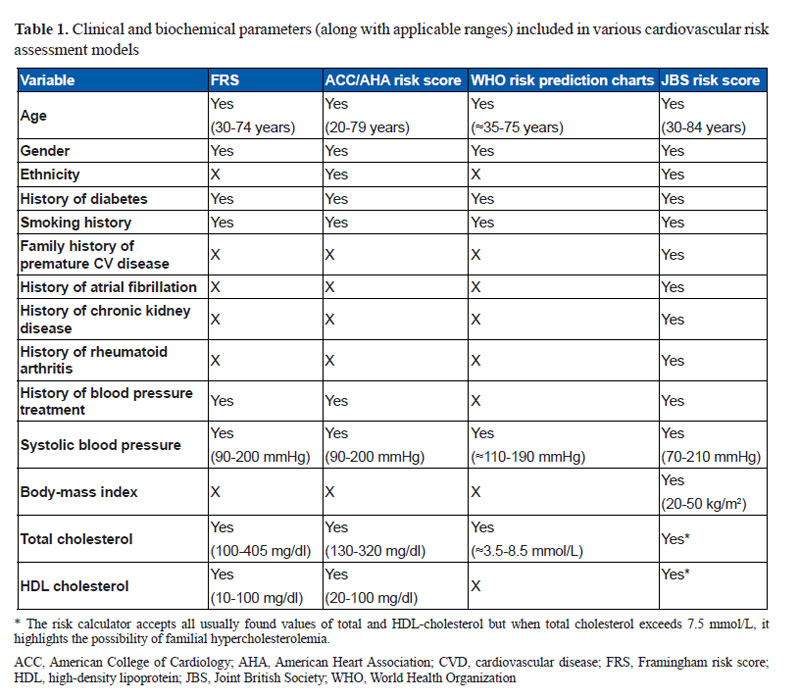
The FRS is based on the data derived from the Framingham Heart Study which was initiated in 1948 in the town of Framingham in Massachusetts, USA. The initial FRS, which was developed in 1998, predicted only coronary heart disease (CHD) risk but subsequently, a new general risk prediction tool was developed in 2008 to predict the overall CV disease risk (1,2). The FRS is based on age, gender, smoking status, diabetes, systolic blood pressure (SBP), total or low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) (Table 1). Based on these parameters, an individual’s 10-year absolute risk of adverse CHD or CV events is estimated.
While FRS has been validated in a number of populations and has been the cornerstone of CV risk assessment over the years, it has several limitations (14). Firstly, it was developed at a time when CVD incidence was at its peak in the US. As a result, FRS tends to overestimate CV risk in populations in which CVD incidence is much lower, such as in the Europeans.Secondly, FRS does not take in to account many of the non-conventional risk factors such as obesity, physical activity, family history of premature CAD, etc which are being increasingly recognized as important contributors to the development of atherosclerotic vascular disease. Finally, FRS relies heavily on age as a determinant of CV risk. Consequently, in a young individual, the estimated 10-year CV risk according to FRS is invariably low, despite the presence of multiple CV risk factors. This has important implications for Indians in whom CVD tends to occur at a younger age than the western populations. As a result, FRS is likely to underestimate CV risk in Indians, as has been amply highlighted in some of the studies done in Indian population (15,16).
Also, as the CV epidemiology has changed dramatically over the last 3-4 decades, it is important to update the risk algorithms incorporating these changes. With this objective, the ACC/AHA task force on CV risk assessment has recently developed a new risk calculator to provide more accurate risk assessment in clinical practice. This new risk calculator, also known as the pooled cohort equations, is based on the data derived from the ARIC (Atherosclerosis Risk in Communities) study, the Cardiovascular Health Study, the CARDIA (Coronary Artery Risk Development in Young Adults) study, and from the Framingham Original and Offspring Study cohorts. It is recommended that the new pooled cohort equations be used in place of FRS for CV risk assessment for clinical purposes. However, the accuracy of risk assessment using this tool, even in the American populations, has become a matter of considerable debate
In the absence of a prospectively validated risk assessment tool for Indians, several different approaches have been explored to provide CV risk assessment in Indian subjects. Chow et al have proposed a calibration method to optimize CV risk estimates for Indians. According to their study, the 10-year risk derived from FRS can be recalibrated by multiplying the calculated risk with a correction factor. For rural Indians, the suggested correction factor is 1.0 for men and 0.8 for women, whereas the same for urban Indians is 1.81 and 1.54 for men and women respectively. However, it has not been prospectively validated and moreover, with FRS itself becoming outdated, the validity of this approach remains questionable.
In 2007, the WHO, in collaboration with the International Society for Hypertension (ISH), published a series of risk prediction charts, each dedicated to a different ethnic-geographic region (4). These risk assessment charts were derived with the help of statistical models using extrapolated data about the prevalence of various CV risk factors in different geographical regions. Although these risk prediction charts have not been systematically validated in prospective studies, they seem to be the only option available for the populations for which prospective studies are not available. JBS3 risk calculator, which is based on QRISK, may be another option. This risk calculator is for use in British populations; but unlike most other risk scores it includes data on Indians also (albeit non-resident Indians) and allows separate risk assessment for people with Indian ethnicity.
Yet another option available is to compare the validity of available risk assessment models in Indians. In a previous study, Kanjilal et al compared 3 different risk scoring systems- FRS, SCORE and an older version of JBS risk score in the family members of the patients with CV disease (16). It was found that despite significantly elevated levels of lipids, pro-inflammatory, pro-thrombotic, and serological markers, all 3 risk scores identified <5% population as being at ‘high-risk’. In a more recent study, our group compared four different risk models in Indian subjects presenting with first MI- FRS, JBS3 risk calculator, WHO/ISH risk prediction charts and the ACC/AHA pooled cohort equations (26). The risk factors included in each of these four different methods have been outlined in Table 1. The study found that the JBS3 risk calculator was the most likely to identify the MI patients has ‘high-risk’ (defined as 10-year risk >20%), ACC/AHA pooled cohort equation and FRS had intermediate accuracy whereas WHO/ISH risk prediction charts were most likely to underestimate the CV risk. A more recent study (yet unpublished data) in outpatients has shown similar findings with JBS3 risk calculator having the best correlation with coronary calcium score. There could be several explanations for greater accuracy of JBS3 risk calculator-it includes data on ethnic Indians, is a more comprehensive risk assessment tool and takes in to account several additional risk factors such as obesity and family history of premature CVD.
A new risk score has also been developed based on the INTERHEART study, which was a cross-sectional study performed in patients recruited from 52 countries (27). This risk score assigns points for each risk factor and based on the total score, the person is categorized as having low, intermediate or high risk of MI. This INTERHEART modifiable risk score (IHMRS) was shown to have good discrimination for the risk of incident MI in the internal validation cohort as well as in the external validation cohort. However, it needs to be noted that IHMRS is not based on prospective data, which remains a major limitation of this tool. In a recent data from the PURE (Prospective Urban Rural Epidemiologic) study, it was found that the mean IHMRS was highest in high-income countries, intermediate in middle-income countries, and lowest in low-income countries but the rates of major CV events were lower in high-income countries than in middle- and low-income countries (28). This itself raises questions about the validity of IHMRS as a useful tool for risk prediction.
While FRS has been validated in a number of populations and has been the cornerstone of CV risk assessment over the years, it has several limitations (14). Firstly, it was developed at a time when CVD incidence was at its peak in the US. As a result, FRS tends to overestimate CV risk in populations in which CVD incidence is much lower, such as in the Europeans.Secondly, FRS does not take in to account many of the non-conventional risk factors such as obesity, physical activity, family history of premature CAD, etc which are being increasingly recognized as important contributors to the development of atherosclerotic vascular disease. Finally, FRS relies heavily on age as a determinant of CV risk. Consequently, in a young individual, the estimated 10-year CV risk according to FRS is invariably low, despite the presence of multiple CV risk factors. This has important implications for Indians in whom CVD tends to occur at a younger age than the western populations. As a result, FRS is likely to underestimate CV risk in Indians, as has been amply highlighted in some of the studies done in Indian population (15,16).

Also, as the CV epidemiology has changed dramatically over the last 3-4 decades, it is important to update the risk algorithms incorporating these changes. With this objective, the ACC/AHA task force on CV risk assessment has recently developed a new risk calculator to provide more accurate risk assessment in clinical practice. This new risk calculator, also known as the pooled cohort equations, is based on the data derived from the ARIC (Atherosclerosis Risk in Communities) study, the Cardiovascular Health Study, the CARDIA (Coronary Artery Risk Development in Young Adults) study, and from the Framingham Original and Offspring Study cohorts. It is recommended that the new pooled cohort equations be used in place of FRS for CV risk assessment for clinical purposes. However, the accuracy of risk assessment using this tool, even in the American populations, has become a matter of considerable debate
CV risk algorithms for use in Indians
Since these risk algorithms are based on epidemiological data, they are applicable only to those populations from which the data has been derived. Unfortunately, none of the currently available risk prediction models is based on Indian data or has been validated in Indians. It is well established that South Asians, including Indians, have increased risk of CV disease as compared to other populations (19-25). Both the genetic make-up and early onset of conventional CV risk factors are believed to contribute to this excess risk (19,21,22). Consistent with this, numerous studies have shown that the risk assessment models developed based on data from the western populations, systematically underestimate the risk in individuals of South Asian origin (20,23,24).
In the absence of a prospectively validated risk assessment tool for Indians, several different approaches have been explored to provide CV risk assessment in Indian subjects. Chow et al have proposed a calibration method to optimize CV risk estimates for Indians. According to their study, the 10-year risk derived from FRS can be recalibrated by multiplying the calculated risk with a correction factor. For rural Indians, the suggested correction factor is 1.0 for men and 0.8 for women, whereas the same for urban Indians is 1.81 and 1.54 for men and women respectively. However, it has not been prospectively validated and moreover, with FRS itself becoming outdated, the validity of this approach remains questionable.
In 2007, the WHO, in collaboration with the International Society for Hypertension (ISH), published a series of risk prediction charts, each dedicated to a different ethnic-geographic region (4). These risk assessment charts were derived with the help of statistical models using extrapolated data about the prevalence of various CV risk factors in different geographical regions. Although these risk prediction charts have not been systematically validated in prospective studies, they seem to be the only option available for the populations for which prospective studies are not available. JBS3 risk calculator, which is based on QRISK, may be another option. This risk calculator is for use in British populations; but unlike most other risk scores it includes data on Indians also (albeit non-resident Indians) and allows separate risk assessment for people with Indian ethnicity.
Yet another option available is to compare the validity of available risk assessment models in Indians. In a previous study, Kanjilal et al compared 3 different risk scoring systems- FRS, SCORE and an older version of JBS risk score in the family members of the patients with CV disease (16). It was found that despite significantly elevated levels of lipids, pro-inflammatory, pro-thrombotic, and serological markers, all 3 risk scores identified <5% population as being at ‘high-risk’. In a more recent study, our group compared four different risk models in Indian subjects presenting with first MI- FRS, JBS3 risk calculator, WHO/ISH risk prediction charts and the ACC/AHA pooled cohort equations (26). The risk factors included in each of these four different methods have been outlined in Table 1. The study found that the JBS3 risk calculator was the most likely to identify the MI patients has ‘high-risk’ (defined as 10-year risk >20%), ACC/AHA pooled cohort equation and FRS had intermediate accuracy whereas WHO/ISH risk prediction charts were most likely to underestimate the CV risk. A more recent study (yet unpublished data) in outpatients has shown similar findings with JBS3 risk calculator having the best correlation with coronary calcium score. There could be several explanations for greater accuracy of JBS3 risk calculator-it includes data on ethnic Indians, is a more comprehensive risk assessment tool and takes in to account several additional risk factors such as obesity and family history of premature CVD.
A new risk score has also been developed based on the INTERHEART study, which was a cross-sectional study performed in patients recruited from 52 countries (27). This risk score assigns points for each risk factor and based on the total score, the person is categorized as having low, intermediate or high risk of MI. This INTERHEART modifiable risk score (IHMRS) was shown to have good discrimination for the risk of incident MI in the internal validation cohort as well as in the external validation cohort. However, it needs to be noted that IHMRS is not based on prospective data, which remains a major limitation of this tool. In a recent data from the PURE (Prospective Urban Rural Epidemiologic) study, it was found that the mean IHMRS was highest in high-income countries, intermediate in middle-income countries, and lowest in low-income countries but the rates of major CV events were lower in high-income countries than in middle- and low-income countries (28). This itself raises questions about the validity of IHMRS as a useful tool for risk prediction.
10-Year Risk Versus Lifetime Risk
The in-use traditional risk algorithms provide risk estimates over a short-term period (i.e. 5 or 10 years) only. For clinical purposes, this approach has served well because the studies evaluating the beneficial impact of various risk reduction strategies have also been of similar duration only. However, it is now being increasingly recognized that many patients who have relatively low short-term CV risk have substantially elevated lifetime risk. This is particularly common in younger individuals because the influence of age is so strong that the short-term risk does not become high until late in the life. The failure to appreciate the value of elevated lifetime CV risk in these individuals is clearly not acceptable as this leads to missing an excellent opportunity to intervene at the right time to prevent CV disease. After all, the goal of primary prevention is to reduce lifetime CV risk, not just 5- or 10-year risk.
Several currently available risk algorithms, including the ACC/AHA pooled cohort equations and the JBS3 risk calculator provide lifetime risk estimates also, in addition to 10-year risk. In addition, the International Atherosclerosis Society has also proposed a simple risk score to estimate lifetime risk (29). It is recommended that lifetime risk be estimated in all individual between 20-59 years of age who are free from CV disease and are not at high short-term risk. If the absolute lifetime risk is 30-44%, it is considered moderately-high and if it is >45%, it is considered high. Aggressive lifestyle measures need to be adopted in all those who have moderately-high or high lifetime CV risk. However, the implications of lifetime risk estimates on initiation of statin therapy are not clear. Although the International Atherosclerosis Society recommends using statins based on lifetime risk estimates, other guidelines do not make any such recommendations because of the issues of cost, risk of side-effects over long-term and lack of clinical trial evidence to show benefit from such a strategy.
Several currently available risk algorithms, including the ACC/AHA pooled cohort equations and the JBS3 risk calculator provide lifetime risk estimates also, in addition to 10-year risk. In addition, the International Atherosclerosis Society has also proposed a simple risk score to estimate lifetime risk (29). It is recommended that lifetime risk be estimated in all individual between 20-59 years of age who are free from CV disease and are not at high short-term risk. If the absolute lifetime risk is 30-44%, it is considered moderately-high and if it is >45%, it is considered high. Aggressive lifestyle measures need to be adopted in all those who have moderately-high or high lifetime CV risk. However, the implications of lifetime risk estimates on initiation of statin therapy are not clear. Although the International Atherosclerosis Society recommends using statins based on lifetime risk estimates, other guidelines do not make any such recommendations because of the issues of cost, risk of side-effects over long-term and lack of clinical trial evidence to show benefit from such a strategy.
Role Of Sub-Clinical Atherosclerosis Imaging
A major limitation of the risk factor based approach is that it works well at the population level but not at the individual level. For example, 20% risk of CV events over 10-years only means that out of 100 such individuals, 20 will develop a vascular event over 10 years, but it is not possible to predict who are those 20 who are going to develop the event. As a result, all 100 patients need to be treated. Although even this ‘over-treatment’ has also been shown to have highly favorable risk-benefit ratio, it will be even more desirable to develop tools that could more accurately identify those who are truly at risk. The assessment of subclinical atherosclerosis is based on this premise only. If a person has evidence of sub-clinical atherosclerosis, he or she has high probability of developing CVD later on, regardless of CV risk factors, and is the most likely to benefit from aggressive preventive strategies. An added advantage of these imaging techniques is that they may also help in improving patient compliance to treatment. The patients, when shown the evidence of ongoing atherosclerosis, are more likely to adopt healthy life-style measures and are more likely to adhere to the pharmacological interventions (30-34).
Several tools for detection of subclinical atherosclerosis are now available- such as carotid plaque assessment, carotid intima-media thickness (CIMT), brachial artery flow-mediated dilatation, coronary calcium score (CCS), ankle-brachial index, pulse wave velocity (PWV), etc. Among them, CCS, carotid ultrasound imaging and PWV have been studied extensively.
Coronary calcium score
CCS is a computed tomography test that detects and quantifies the amount of calcium in the coronary arteries. As calcium is deposited in coronary arteries only in the atherosclerotic plaques, the presence of coronary calcium serves as a direct evidence of ongoing coronary atherosclerosis. The total CCS is a measure of total atherosclerotic burden in the coronaries and has an excellent correlation with the risk of adverse CV events. The CCS estimation involves radiation exposure but does not require the use of iodinated contrast medium (35).
A large number of studies have evaluated the role of CCS in prediction of CV risk in asymptomatic individuals. A meta-analysis involving six large, prospective studies with a total of nearly 30,000 subjects showed that the relative risk of CVD death or MI over 3–5 years’ follow-up was 4.3 in subjects with any measurable calcium compared to those with zero CCS and the absolute risk of events was 1.91% and 0.4% per year, respectively (35). Further, there was a graded relationship between increasing CCS and increasing risk of vascular events with the annual event rates being 0.7%, 2.1%, 4.6% and 7.1% in patients with CCS 1–112, 100–400, 400–999 and ≥1000, respectively.
In addition, it has also been shown that CCS provides prognostic information that is incremental to the information provided by the conventional CV risk factors, FRS, and some of the newer risk factors such as high-sensitivity C-reactive protein and body-mass index (36-41). The incremental benefit is maximum in patients with intermediate FRS (i.e. 10-year event rate 10–20%) in whom CCS <100, 100–399 and ≥400 have been shown to confer 0.4%, 1.3% and 2.4% risk of CV events per year, respectively. The more recent Multi-ethnic study of atherosclerosis (MESA) reconfirmed the value of CCS in prediction of CV risk. The highest quartile CCS values had the hazard ratio of 8.2 for coronary heart disease events as compared to the values in quartiles 1 and 2 (42).
The experience with CCS in Indian patients is limited with only few studies published so far (43,44). However, these studies have confirmed that CCS correlates with the extent of coronary artery disease (CAD) in Indian subjects as well.
Carotid ultrasound imaging
Carotid ultrasound imaging is based on the premise that atherosclerosis is a generalized process and therefore, the evidence of atherosclerosis in carotid arteries is likely to indicate high risk of coronary events also. This hypothesis has been adequately validated in autopsy studies as well as in a number of large clinical trials (45).
Both, carotid plaques and CIMT can be evaluated by carotid ultrasound imaging. CIMT refers to the combined thickness of intima and media of the carotid arteries, usually measured at the distal common carotid artery. A large number of clinical trials have shown that increased CIMT is associated with increased risk of vascular events, independent of conventional CV risk factors or FRS (45). A meta-analysis of 8 large CIMT studies that had enrolled 37197 subjects with a mean follow-up duration of 5.5 years was published recently. It showed that for an absolute CIMT difference of 0.1 mm, the future risk of MI increased by 10-15% and the stroke risk increased by 13-18% (46). The evidence with carotid plaques is less extensive but suggests that the presence of a carotid plaque has stronger predictive accuracy than CIMT. In a prospective, 10-year follow-up study, 13221 low-risk, healthy, asymptomatic individuals were included and carotid and femoral plaques were evaluated by B-mode ultrasound (47). The study was concluded when 10000 subjects completed the 10-year follow-up. It was found that at 10 years, there were 10 events (out of 7989 subjects) in patients with normal carotid and femoral ultrasound, 81 events (930 subjects; incidence=8.6%) in those with increased carotid or femoral IMT, 239 events (611 subjects; 39.3%) in those with non-stenotic plaques and 381 events (470 subjects; 81.1%) in those with stenotic plaques (Figure 1). Together, carotid and femoral IMT and plaque assessment identified 2011 subjects (20.1% of the population) who accounted for 98.6% of all CV events and deaths in the following 10 years. Given that this study had included patients who were otherwise at low-risk, it provided strong evidence supporting the value of IMT and plaque assessment and also demonstrated that the presence of plaque had greater prognostic significance than elevated IMT alone. However, a recent metaanalysis has suggested that even though CIMT had incremental value over conventional risk factors, the net reclassification was not significant enough for routine use (48).
.jpg)
A number of studies have evaluated the role of CIMT in Indians also but all of them were cross-sectional (49-57). Nevertheless, these studies have demonstrated that in Indian subjects too, CIMT was related to CV risk factors, presence of CAD and the angiographic extent of CAD.
How to apply these tools in clinical practice?
Both, carotid plaques and CIMT can be evaluated by carotid ultrasound imaging. CIMT refers to the combined thickness of intima and media of the carotid arteries, usually measured at the distal common carotid artery. A large number of clinical trials have shown that increased CIMT is associated with increased risk of vascular events, independent of conventional CV risk factors or FRS (45). A meta-analysis of 8 large CIMT studies that had enrolled 37197 subjects with a mean follow-up duration of 5.5 years was published recently. It showed that for an absolute CIMT difference of 0.1 mm, the future risk of MI increased by 10-15% and the stroke risk increased by 13-18% (46). The evidence with carotid plaques is less extensive but suggests that the presence of a carotid plaque has stronger predictive accuracy than CIMT. In a prospective, 10-year follow-up study, 13221 low-risk, healthy, asymptomatic individuals were included and carotid and femoral plaques were evaluated by B-mode ultrasound (47). The study was concluded when 10000 subjects completed the 10-year follow-up. It was found that at 10 years, there were 10 events (out of 7989 subjects) in patients with normal carotid and femoral ultrasound, 81 events (930 subjects; incidence=8.6%) in those with increased carotid or femoral IMT, 239 events (611 subjects; 39.3%) in those with non-stenotic plaques and 381 events (470 subjects; 81.1%) in those with stenotic plaques (Figure 1). Together, carotid and femoral IMT and plaque assessment identified 2011 subjects (20.1% of the population) who accounted for 98.6% of all CV events and deaths in the following 10 years. Given that this study had included patients who were otherwise at low-risk, it provided strong evidence supporting the value of IMT and plaque assessment and also demonstrated that the presence of plaque had greater prognostic significance than elevated IMT alone. However, a recent metaanalysis has suggested that even though CIMT had incremental value over conventional risk factors, the net reclassification was not significant enough for routine use (48).
.jpg)
A number of studies have evaluated the role of CIMT in Indians also but all of them were cross-sectional (49-57). Nevertheless, these studies have demonstrated that in Indian subjects too, CIMT was related to CV risk factors, presence of CAD and the angiographic extent of CAD.
Aortic pulse wave velocity
PWV is a measure of arterial stiffness. With the availability of several non-invasive devices such as Complior®, SphygmoCor®, Periscope®, etc, it has become feasible to easily measure arterial PWV in clinical practice. Using these devices, PWV can be measured in different vascular segments, but the maximum evidence has been generated using either carotid-femoral or brachial-ankle PWV (58).
Aortic stiffness, measured as carotid-femoral or brachial-ankle PWV, has been shown to have excellent predictive value for CV mortality, total mortality, fatal and non-fatal coronary events and fatal strokes in different patient subsets including hypertensives (59,60), diabetics (61), elderly subjects (62,63), patients with end-stage renal disease (64) as well as in the general population (65,66). Further, in many of these studies, PWV was found to have independent predictive value over conventional CV risk factors either considered alone or in combination in the form of Framingham risk score. However, it is important to note that arterial stiffness is more of a manifestation of arteriosclerosis, than atherosclerosis, and therefore, PWV appears to be the most valuable in the evaluation of pathophysiological states associated with arteriosclerosis, such as hypertension, ageing and end-stage renal disease.
A few studies have assessed arterial stiffness in Indian subjects also and have demonstrated a significant relationship between PWV and CV risk factors and incident CV disease (52).
How to apply these tools in clinical practice?
At present, CCS is clearly the most robust tool for assessment of presence of subclinical atherosclerosis and has the greatest incremental value for predicting risk of occurrence of CV events. However, CCS is expensive, not readily available and carries a small but definite risk of radiation exposure. These issues, which are of even greater concern for a country like ours, markedly limit the utility of CCS in regular practice. In contrast, CIMT and PWV are simple, relatively inexpensive, have wider availability, and most importantly, are completely safe. Considering these issues, it may seem reasonable to use these modalities in a hierarchical manner, with CIMT and PWV being the first-line investigations and CCS being the second-line option. In fact, in a recent study, our group has demonstrated that a combination of normal CIMT and normal PWV has very high negative predictive value for incident coronary atherosclerosis (67). Thus, combining these two modalities may allow identification of those individuals who are at very low-risk of having vascular events. On the contrary, if both are abnormal or if a carotid plaque is found, it would indicate sufficiently high vascular risk to warrant further evaluation.
Other Markers For CV Risk Assessment
Numerous other clinical and biochemical markers have been evaluated for their role in prediction of CV risk. These include high-sensitive c-reactive protein (hsCRP), lipoprotein a [Lp(a)], apolipoproteins, inflammatory cytokines, fibrinogen, etc. Atherosclerosis is now well-recognized to be an inflammatory disease. Of all the inflammatory markers, hsCRP has been studied the most extensively (68-72). Several large-scale prospective studies have shown that elevated hsCRP levels strongly predict the risk of CV events and may be a target for initiation of statin therapy, irrespective of the lipid levels(72). However, its use has several practical limitations. The assays for hsCRP and not adequately standardized, not readily available and are expensive. Moreover, hsCRP, being a marker of inflammation, is easily affected by any inflammatory condition and does no longer remain an accurate marker of atherosclerotic process.
Lp(a) is another biochemical marker which has particular relevance for Indians. Indians are known to have higher levels of Lp (a) with as many as 30–40% Indians having levels >20 mg/dL, which is generally considered as the threshold for high risk for CAD (73). Lp(a) is a genetically modified form of LDL-C particle and has greater propensity, than LDL-C, to bind to oxidized lipoproteins. As a result, elevated Lp(a) levels accentuate the risk imparted by several other CV risk factors such as diabetes, low HDL-C and high LDL-C (74). A number of studies have reported an association between Lp (a) levels and incident CV disease (75-77), though the prospective studies have failed to conclusively establish a causative link between Lp (a) and CV disease (78-80).
.jpg)
Lp(a) is another biochemical marker which has particular relevance for Indians. Indians are known to have higher levels of Lp (a) with as many as 30–40% Indians having levels >20 mg/dL, which is generally considered as the threshold for high risk for CAD (73). Lp(a) is a genetically modified form of LDL-C particle and has greater propensity, than LDL-C, to bind to oxidized lipoproteins. As a result, elevated Lp(a) levels accentuate the risk imparted by several other CV risk factors such as diabetes, low HDL-C and high LDL-C (74). A number of studies have reported an association between Lp (a) levels and incident CV disease (75-77), though the prospective studies have failed to conclusively establish a causative link between Lp (a) and CV disease (78-80).
.jpg)
Approach To CV Risk Assessment For Primary Prevention In Indians
Figure 2 outlines a suggested step-wise approach to CV risk assessment in Indian subjects without pre-existing CV disease.
In brief, presence of any one or more of the following should indicate high CV risk (>20% risk of hard vascular events over 10-years)-
- Long-standing diabetes
- Diabetes with target organ damage or with multiple other CV risk factors
- Chronic kidney disease
- Extreme of a single major risk factor [i.e. heavy smoker, strong family history of premature CV disease, Lp(a) >50 mg/dL]
- Evidence of subclinical atherosclerosis in the form of CCS >300 or elevated both CIMT and PWV or presence of a carotid plaque
- Estimated 10-year CV risk >20% (WHO/ISH risk prediction charts or JBS3 risk calculator are preferred)
- Estimated 10-year CV risk 10-20% with one or more non-conventional CV risk factors (e.g. obesity, metabolic syndrome, impaired fasting glucose/impaired glucose tolerance, elevated hsCRP, etc.
- If none of these conditions is present, then a person is considered to be at intermediate risk (10-20%) of vascular events if-
- The estimated 10-year CV risk is 10-20% but there is no apparent non-convention CV risk factor
- Only early evidence of subclinical atherosclerosis is present (i.e. CCS 100-299 or only CIMT or PWV is increased and there is no carotid plaque)
- All diabetics excluding those included in the high-risk category as mentioned above
- Those with Lp(a) 20-49 mg/dL and no other high-risk features
Conclusions
Estimation of CV risk may appear a time-consuming process but is a worthwhile exercise. In the current era of evidence-based medicine, all our decisions need to be based on robust scientific evidence and not on personal preferences. This is especially true for primary prevention where risk benefit ratio may not be that favorable for many pharmacotherapies (for example, aspirin), and even for relatively low risk therapies, the issues of cost, side-effects and adverse social beliefs and practices needs to be considered. The current guidelines recommend that the use of stains and aspirin should be linked to the anticipated CV risk in the given individual. Hence, it is essential to assess CV risk in every patient in whom a statin or aspirin is prescribed. The estimation of CV risk has an added advantage that it provides an objective measure of the ‘seriousness’ of the illness and may help improve patient compliance to the treatment.
References
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47.
- D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB.. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-53.
- Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation 2002;105:310-5.
- World Health Organization. Prevention of Cardiovascular Disease Guidelines for assessment and management of cardiovascular risk. Geneva: WHO. Geneva: WHO, 2007.
- Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, Njølstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM; SCORE project group.. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987-1003.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, Brindle P. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475-82.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ 2007;335:136.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Brindle P. Performance of the QRISK cardiovascular risk prediction algorithm in an independent UK sample of patients from general practice: a validation study. Heart 2008;94:34-9.
- Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. Jama 2007;297:611-9.
- Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation 2008;118:2243-51, 4p following 2251.
- Greenland P, Smith SC, Jr., Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation 2001;104:1863-7.
- Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines.. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S49-73.
- Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014;100 Suppl 2:ii1-ii67.
- Chia YC. Review of tools of cardiovascular disease risk stratification: interpretation, customisation and application in clinical practice. Singapore Med J 2011;52:116-23.
- Bansal M, Shrivastava S, Mehrotra R, Agarwal V, Kasliwal RR. Low Framingham risk score despite high prevalence of metabolic syndrome in asymptomatic North-Indian population. J Assoc Physicians India 2009;57:17-22.
- Kanjilal S, Rao VS, Mukherjee M, Natesha BK, Renuka KS, Sibi K, Iyengar SS, Kakkar VV. Application of cardiovascular disease risk prediction models and the relevance of novel biomarkers to risk stratification in Asian Indians. Vasc Health Risk Manag 2008;4:199-211.
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013;382:1762-5.
- Amin NP, Martin SS, Blaha MJ, Nasir K, Blumenthal RS, Michos ED. Headed in the Right Direction But at Risk for Miscalculation: A Critical Appraisal of the 2013 ACC/AHA Risk Assessment Guidelines. J Am Coll Cardiol 2014;63:2789-2794.
- Enas EA, Garg A, Davidson MA, Nair VM, Huet BA, Yusuf S. Coronary heart disease and its risk factors in first-generation immigrant Asian Indians to the United States of America. Indian Heart J 1996;48:343-353.
- Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, Kelemen L, Yi C, Lonn E, Gerstein H, Hegele RA, McQueen M. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet 2000;356:279-84.
- McKeigue PM, Miller GJ, Marmot MG. Coronary heart disease in south Asians overseas: a review. J Clin Epidemiol 1989;42:597-609.
- Joshi P, Islam S, Pais P, Reddy S, Dorairaj P, Kazmi K, Pandey MR, Haque S, Mendis S, Rangarajan S, Yusuf S. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA 2007;297:286-94.
- Perumal L, Wells S, Ameratunga S, Pylypchuk RD, Elley CR, Riddell T, Kerr A, Crengle S, Gentles D, Jackson R. Markedly different clustering of CVD risk factors in New Zealand Indian and European people but similar risk scores (PREDICT-14). Aust N Z J Public Health 2012;36:141-4.
- Bhopal R, Fischbacher C, Vartiainen E, Unwin N, White M, Alberti G. Predicted and observed cardiovascular disease in South Asians: application of FINRISK, Framingham and SCORE models to Newcastle Heart Project data. Journal of public health (Oxford, England) 2005;27:93-100.
- Liem SS, Oemrawsingh PV, Cannegieter SC, Le Cessie S, Schreur J, Rosendaal FR, Schalij MJ. Cardiovascular risk in young apparently healthy descendents from Asian Indian migrants in the Netherlands: the SHIVA study. Neth Heart J 2009;17:155-61.
- Bansal M, Kasliwal RR, Trehan N. Comparative accuracy of different risk scores in assessing cardiovascular risk in Indians: A study in patients with first myocardial infarction. Indian Heart J. 2014;66:580-6.
- McGorrian C, Yusuf S, Islam S, Jung H, Rangarajan S, Avezum A, Prabhakaran D, Almahmeed W, Rumboldt Z, Budaj A, Dans AL, Gerstein HC, Teo K, Anand SS; INTERHEART Investigators. Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J 2011;32:581-9.
- Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G; PURE Investigators. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 2014;371:818-27.
- International Atherosclerotic Society Position Paper: Global Recommendations for the Management of Dyslipidemia 2013. Available at http://www.athero.org/IASPositionPaper.asp. Last accessed 21st March 2015.
- Bovet P, Perret F, Cornuz J, Quilindo J, Paccaud F. Improved smoking cessation in smokers given ultrasound photographs of their own atherosclerotic plaques. Prev Med 2002;34:215-20.
- Wyman RA, Gimelli G, McBride PE, Korcarz CE, Stein JH. Does detection of carotid plaque affect physician behavior or motivate patients? Am Heart J 2007;154:1072-7.
- Barth JD. Which tools are in your cardiac workshop? Carotid ultrasound, endothelial function, and magnetic resonance imaging. Am J Cardiol 2001;87:8A-14A.
- O’Malley PG, Feuerstein IM, Taylor AJ. Impact of electron beam tomography, with or without case management, on motivation, behavioral change, and cardiovascular risk profile: a randomized controlled trial. JAMA 2003;289:2215-23.
- Orakzai RH, Nasir K, Orakzai SH, Kalia N, Gopal A, Musunuru K, Blumenthal RS, Budoff MJ. Effect of patient visualization of coronary calcium by electron beam computed tomography on changes in beneficial lifestyle behaviors. Am J Cardiol 2008;101:999-1002.
- Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS; American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography); Society of Atherosclerosis Imaging and Prevention; Society of Cardiovascular Computed Tomography. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2007;49:378-402.
- Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 2004;291:210-5.
- Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol 2005;46:807-14.
- Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol 2005;46:158-65.
- Kondos GT, Hoff JA, Sevrukov A, Daviglus ML, Garside DB, Devries SS, Chomka EV, Liu K. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation 2003;107:2571-6.
- Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation 2005;112:572-7.
- LaMonte MJ, FitzGerald SJ, Church TS, Barlow CE, Radford NB, Levine BD, Pippin JJ, Gibbons LW, Blair SN, Nichaman MZ. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol 2005;162:421-9.
- Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 2008;168:1333-9.
- Shrivastava S1, Agrawal V, Kasliwal RR, Jangid DR, Sen A, Verma A, Trehan N. Coronary calcium and coronary artery disease: an Indian perspective. Indian Heart J 2003;55:344-8.
- Wasnik A, Raut A, Morani A. Coronary calcium scoring in asymptomatic Indian population: correlation with age, gender and risk factors--a prospective study on 500 subjects. Indian Heart J 2007;59:232-8.
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS; American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Disease Risk: A Consensus Statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93-111.
- Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459-67.
- Belcaro G, Nicolaides AN, Ramaswami G, Cesarone MR, De Sanctis M, Incandela L, Ferrari P, Geroulakos G, Barsotti A, Griffin M, Dhanjil S, Sabetai M, Bucci M, Martines G. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study(1)). Atherosclerosis 2001;156:379-87.
- Wald DS, Bestwick JP, Morton G, Drummond L, Jenkins N, Khodabakhsh P, Curzen NP. Combining carotid intima-media thickness with carotid plaque on screening for coronary heart disease. J Med Screen 2009;16:155-9.
- Hansa G, Bhargava K, Bansal M, Tandon S, Kasliwal RR. Carotid intima-media thickness and coronary artery disease: an Indian perspective. Asian Cardiovasc Thorac Ann 2003;11:217-21.
- Kasliwal RR, Bansal M, Gupta H, Agrawal S. Association of carotid intima-media thickness with left main coronary artery disease. Indian Heart J 2007;59:50-5.
- Kasliwal RR, Agrawal S, Bansal M. Carotid Intima-Media Thickness and Brachial Artery Flow-Mediated Dilatation in Patients with and without Metabolic Syndrome. Indian Heart J 2006;58:42-6.
- Kasliwal RR, Bansal M, Bhargava K, Gupta H, Tandon S, Agrawal V. Carotid intima-media thickness and brachial-ankle pulse wave velocity in patients with and without coronary artery disease. Indian Heart J 2004;56:117-22.
- Ravikumar R, Deepa R, Shanthirani C, Mohan V. Comparison of carotid intima-media thickness, arterial stiffness, and brachial artery flow mediated dilatation in diabetic and nondiabetic subjects (The Chennai Urban Population Study [CUPS-9]). Am J Cardiol 2002;90:702-7.
- Jadhav UM, Kadam NN. Association of microalbuminuria with carotid intima-media thickness and coronary artery disease--a cross-sectional study in Western India. J Assoc Physicians India 2002;50:1124-9.
- Rema M, Mohan V, Deepa R, Ravikumar R. Association of carotid intima-media thickness and arterial stiffness with diabetic retinopathy: the Chennai Urban Rural Epidemiology Study (CURES-2). Diabetes Care 2004;27:1962-7.
- Chow CK, McQuillan B, Raju PK, Iyengar S, Raju R, Harmer JA, Neal BC, Celermajer DS. Greater adverse effects of cholesterol and diabetes on carotid intima-media thickness in South Asian Indians: comparison of risk factor-IMT associations in two population-based surveys. Atherosclerosis 2008;199:116-22.
- Ahmad J, Ahmned F, Siddiqui MA, Khan AR, Katyal P, Hameed B, Ahmad I. Inflammatory markers, insulin resistance and carotid intima-media thickness in North-Indian type 2 diabetic subjects. J Assoc Physicians India 2007;55:693-9.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588-605.
- Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002;39:10-5.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001;37:1236-41.
- Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002;106:2085-90.
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006;113:657-63.
- Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A; Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005;111:3384-90.
- Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999;99:2434-9.
- Shokawa T, Imazu M, Yamamoto H, Toyofuku M, Tasaki N, Okimoto T, Yamane K, Kohno N. Pulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J 2005;69:259-64.
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006;113:664-70.
- Kasliwal RR, Bansal M, Mehrotra R, Ahlawat K, Trehan N. Comparative Diagnostic Accuracy of Different Measures of Preclinical Atherosclerosis for Detection of Atherosclerotic Coronary Artery Disease. J Clin Prev Cardiol 2014;3:36-42.
- 68. Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004;351:2599-610.
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;347:1557-65.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836-43.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973-9.
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973-9.
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195-207.
- Misra A. Atherosclerosis in Indians and lipoprotein (a). J Assoc Physicians India 1999;47:313-7.
- Enas EA, Singh V, Munjal YP, Bhandari S, Yadave RD, Manchanda SC. Reducing the burden of coronary artery disease in India: challenges and opportunities. Indian Heart J 2008;60:161-75.
- Stubbs P, Seed M, Lane D, Collinson P, Kendall F, Noble M. Lipoprotein(a) as a risk predictor for cardiac mortality in patients with acute coronary syndromes. Eur Heart J 1998;19:1355-64.
- Seman LJ, DeLuca C, Jenner JL, Cupples LA, McNamara JR, Wilson PW, Castelli WP, Ordovas JM, Schaefer EJ. Lipoprotein(a)-cholesterol and coronary heart disease in the Framingham Heart Study. Clin Chem 1999;45:1039-46.
- Budde T, Fechtrup C, Bösenberg E, Vielhauer C, Enbergs A, Schulte H, Assmann G, Breithardt G. Plasma Lp(a) levels correlate with number, severity, and length-extension of coronary lesions in male patients undergoing coronary arteriography for clinically suspected coronary atherosclerosis. Arterioscler Thromb 1994;14:1730-6.
- Nishino M, Malloy MJ, Naya-Vigne J, Russell J, Kane JP, Redberg RF. Lack of association of lipoprotein(a) levels with coronary calcium deposits in asymptomatic postmenopausal women. J Am Coll Cardiol 2000;35:314-20.
- Moliterno DJ, Jokinen EV, Miserez AR, Lange RA, Willard JE, Boerwinkle E, Hillis LD, Hobbs HH. No association between plasma lipoprotein(a) concentrations and the presence or absence of coronary atherosclerosis in African-Americans. Arterioscler Thromb Vasc Biol 1995;15:850-5.
- Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation 2000;102:1082-5.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528


