The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Review Article
Atrial Fibrillation Part II: Rate and Rhythm-Control Strategies of Management
Volume 3, Jul 2014
Kartikeya Bhargava, MD, DNB, Gurgaon, India
J Clin Prev Cardiol. 2014;3(3):85-92
IntroductionThe major goals of management of atrial fibrillation (AF) include the following:
- Prevention and treatment of symptoms related to AF
- Prevention of development or worsening of left ventricular dysfunction and heart failure
- Prevention of stroke due to AF
Rate-control Strategy
One of the foremost problems with AF is the rapid ventricular rate that develops in most patients with intact and normal atrioventricular (AV) conduction. This results not only in AF-related symptoms like palpitations, dyspnea, and occasionally chest pain but may also result in development of left ventricular dysfunction (tachycardiomyopathy) and heart failure in some patients. These symptoms can be treated or prevented by using the rate-control strategy that aims at controlling the ventricular rate without achieving sinus rhythm. Rate-control strategy is often the initial strategy in most patients with hemodynamically stable AF and improves the quality of life and reduces morbidity.
Means of achieving rate control
Drugs. Many drugs that include the AV nodal blocking agents like β-blockers, nondihydropyridine calcium channel blockers, digoxin, and certain anti-arrhythmic drugs like amiodarone have been used and evaluated as rate-controlling agents in AF.
AV nodal ablation. Radiofrequency ablation of AV node or AV junction to produce iatrogenic complete AV block along with permanent ventricular pacemaker implantation is a very effective means of rate control. However, since it makes the patient pacemaker-dependent, and long-term right ventricular pacing may also cause left ventricular dysfunction in some patients, this strategy should be reserved for patients in whom rate control with drugs is inadequate and rhythm control is not achievable (1,2). The patients undergoing AV junctional ablation who have left ventricular systolic dysfunction or those who are likely to develop left ventricular dysfunction by right ventricular pacing, may benefit from biventricular pacing instead of right ventricular pacing in the long term (3).
Drug therapy for rate control
Many drugs can be used to achieve rate control in patients with AF, though β-blockers are preferred in most situations (4). Intravenous route may be used if rapid control of ventricular rate during AF is required as in patients with marked symptoms or ongoing ischemia or hypotension (5). Alternatively, electrical cardioversion is preferred in these situations, though risk of stroke exists if the patient is not or inadequately anticoagulated. In case of hemodynamic instability due to rapid ventricular rate in AF, electrical cardioversion should be performed urgently irrespective of prior anticoagulation status.
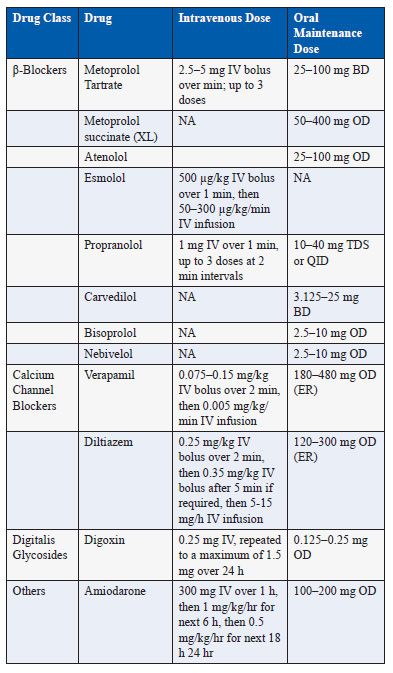
The common drugs used for rate control in AF with their oral and intravenous doses are listed in Table 1.
Table 1. Drugs used for rate control in atrial fibrillation Specific drugs for rate control.
β-Blockers. These agents slow the AV nodal conduction by causing sympathetic blockage. Some of the agents can also be used intravenously. They are the most effective and most commonly used drugs for rate control in AF (4). They are the first-line agents in patients with coexisting coronary artery disease or heart failure. However, β-blockers are relatively contraindicated in patient with severe obstructive airway disease. They can be combined with other agents like digoxin if rate control is inadequate and there dose should be titrated to prevent excessive bradycardia (6).
Calcium Channel Blockers. The non-dihydropyridine calcium channel blockers that include diltiazem and verapamil block the L-type calcium channels with resultant direct AV nodal blocking effects. These agents should not be used in patients with left ventricular systolic dysfunction and decompensated heart failure since their negative inotropic effects can cause more harm.
Digoxin. Although digoxin is commonly used for rate control, it is not the preferred drug because of its ineffectiveness to control the ventricular rate during exercise (6). The intravenous digoxin has a slow onset and late peak of action and hence it cannot achieve rapid rate control in semiemergent situations. Moreover, it has a narrow therapeutic window and many drug interactions. It should be used with caution with reduced dose in elderly patients, patients with renal dysfunction, and with concomitant use of drugs like amiodarone that increase its plasma levels. Despite these limitations, digoxin can be used for rate control along with β-blockers and especially in patients with heart failure where its lack of negative inotropic effect comes as an advantage.
Rhythm-control Strategy
The management of AF with means to restore and maintain sinus rhythm over long term is known as the rhythm-control strategy. Many trials including large randomized trials that have compared rhythm (using drugs and cardioversion) and rate (using drugs and AV junction ablation) control strategies in patients with AF have failed to show a superiority of rhythm control in terms of mortality (4,7). Moreover, the rhythm-control strategy may require more hospitalizations essentially due to more attempts at electrical cardioversion. However, this does not mean that achievement and maintenance of sinus rhythm over long term in patients with AF is not beneficial. In fact, in the same studies patients who maintained sinus rhythm over long term faired much better compared to those who remained in AF. It essentially suggests that the means (drugs) of achieving and maintaining sinus rhythm are not effective enough and their side effects in the long term outweigh any benefit gained from attempt at achieving long-term sinus rhythm.
Means of achieving rhythm control
- Drug therapy – In rhythm control strategy, drugs may be used in the following roles:
- Drug therapy for pharmacological cardioversion – anti-arrhythmic drugs (AADs) may be used for attempted cardioversion of AF to sinus rhythm.
- Drug therapy for maintenance of sinus rhythm – long-term use of AADs to maintain sinus rhythm and prevent recurrence of AF once the rhythm is restored by electrical or pharmacological cardioversion
- Upstream drug therapy – use of non-AADs to prevent recurrence of AF. These include angiotensin-converting-enzyme (ACE) inhibitors, angiotensin receptor blockers, statins, and n-3 polyunsaturated fatty acids. These agents modify the atrial substrate by stopping or delaying the cellular processes leading to AF thereby reducing the susceptibility and/or progression of AF. The efficacy of these agents is not established and remains controversial (8,9).
- Electrical cardioversion – This procedure entails delivery of direct-current electric shock to the chest wall after appropriate sedation and anticoagulation measures to convert AF to sinus rhythm. The shock is synchronized to the QRS complex to prevent its delivery in the vulnerable phase (T-wave) of ventricular repolarization that may result in ventricular fibrillation.
- Catheter ablation for AF – Ablation for AF is a catheter-based procedure wherein the triggers of AF in the pulmonary veins or other sites are ablated using radiofrequency or other energy sources with or without additional ablation in the atria to modify the substrate. The AF ablation procedure has evolved since its initial description when it was found that AF results from electrical firing (triggers) from the pulmonary veins that can be isolated using radiofrequency ablation to achieve and maintain sinus rhythm in many selected patients. Although, still not perfect, the efficacy of RF ablation for AF for maintaining sinus rhythm is superior to the currently available anti-arrhythmic drug therapy.
- Pacemaker therapy – The pacemaker may be required in patients with AF who also have symptomatic bradycardia often due to underlying sinus node dysfunction.
- Surgical ablation for AF – Surgical ablation for AF is performed concomitantly with other cardiac surgery like coronary bypass surgery/valve surgery or uncommonly as a stand-alone AF surgery. These can be either conventional “cut and sew” procedures or more commonly may utilize radiofrequency or cryoablation to replicate the surgical lines of ablation. The results and outcomes of these procedures vary according to the operator experience, institutional practice, underlying disease duration, and severity.
Direct-current Cardioversion
The electric shock given during direct-current cardioversion (DCCV) results in simultaneous momentary depolarization of all atrial myocardial cells, thus allowing the sinus node to resume normal pacemaker activity. It is the most effective modality to convert AF to sinus rhythm, though AF may recur in many patients either immediately or some time later. Since it is painful, appropriate conscious sedation under supervision of anesthetist is essential as also monitoring of oxygen saturation and blood pressure. Commonly used drugs for sedation include midazolam, fentanyl, and propofol all of which are short-acting allowing for full consciousness to return after few minutes.
DCCV should not be performed in patients with severe electrolyte imbalance especially hypokalemia or in digoxin toxicity due to risk of intractable ventricular arrhythmias. The risks and complications associated with DCCV include sedation-related complications (hypoxia, aspiration, etc.), thromboembolism, arrhythmias like ventricular tachycardia or fibrillation or bradyarrhythmias, muscle pain, and skin burn.
The risk of thromboembolism is due to migration of a clot present in the left atrium or left atrial appendage (LAA) on resumption of the mechanical contractile function of atria on restoration of sinus rhythm. The risk of thromboembolism post DCCV is highest in the initial 72 h and maximum in the first 7–10 days (10,11). However, post-cardioversion, the atrial mechanical function may not return (stunning) for up to 4 weeks despite sinus rhythm and thus clot formation can occur during this period. Hence, anticoagulation is necessary for at least 4 weeks after DCCV (12,13). The anticoagulation for at least 3 weeks prior to DCCV is necessary to ensure that no LAA clot is present at the time of the procedure. Based on peri-procedure anticoagulation, the DCCV can be performed by two strategies:
- Conventional strategy involves anticoagulation 3 weeks prior and 4 weeks post-cardioversion (12).
- Trans-esophageal echo (TEE) guided strategy involves performance of TEE to rule out LAA clot
- just prior to DCCV and anticoagulation for at least 4 weeks post-cardioversion (14,15). If there is a clot in LAA on TEE, anticoagulation for 3–4 weeks to dissolve the clot, followed by confirmation by TEE, is essential before cardioversion can be considered. Anticoagulation in post- cardioversion period of at least 4 weeks is essential even if there was no LAA clot in pre-procedural TEE due to risk of thrombus formation in stunned atria.
During DCCV of first episode of AF lasting clearly <48 h, antecedent anticoagulation or TEE is not necessary since the risk of thrombus formation in such short-lasting AF is minimal (16). Similarly, patients with hemodynamically unstable AF should undergo DCCV irrespective of antecedent anticoagulation status and without waiting for TEE.
Pharmacological Cardioversion
The drugs that can be used for conversion of AF to sinus rhythm are listed in Table 2 (17,18). The efficacy of these drugs in restoring sinus rhythm is at the best modest ranging from 30% to 50%. Shorter the duration of AF, better is the efficacy with best results in AF of less than 7 days duration. The major risk with these drugs is QT prolongation related polymorphic ventricular tachycardia or torsades de pointes that occurs in 3–4% of patients (19). The risk may persist even up to 4–6 h after cardioversion. Among these agents, vernakalant or ibutilide probably have higher efficacy and least side effects but unfortunately both are not available in India. Even if these agents do not restore sinus rhythm, they can improve the efficacy of DCCV (19).
Single oral dose of flecainide or propafenone may be used after pretreatment with a β-blocker or calcium channel blocker as a “pill in the pocket” approach to restore normal rhythm shortly after onset of symptoms of AF in patients with paroxysmal AF (20,21).
It should be remembered that the risk of thromboembolism during pharmacological cardioversion is same as during electrical cardioversion and similar anticoagulation guidelines need to be adhered. This is due to the fact that the risk of embolism is related to the resumption of the mechanical contractile function of the atrium and not the mechanical effect of shock dislodging the clot.
Newer atrial selective drugs that can restore sinus rhythm with much lower risk of ventricular proarrhythmia are in various stages of development. Vernakalant is one such drug that has been approved in Europe for acute conversion of AF to sinus rhythm.
Table 2. Drugs for pharmacological cardioversion of atrial fibrillation
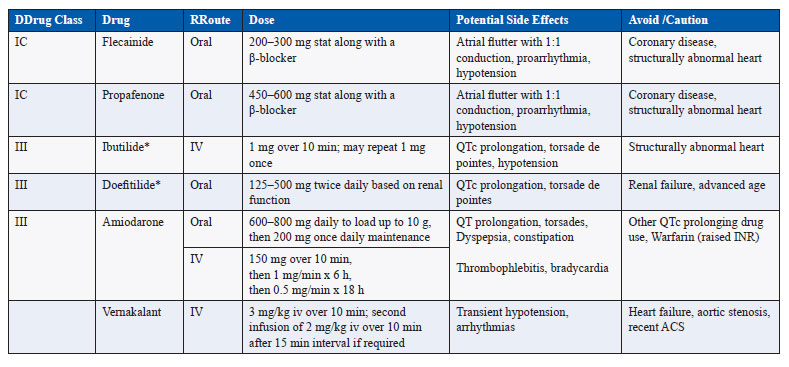
*Not available in India
Drug Therapy for Maintenance of Sinus Rhythm
Once sinus rhythm has been restored by electrical or pharmacological cardioversion, it is desirable to maintain sinus rhythm for as long as possible. The chance of recurrence of AF remains high especially if there is underlying cause that has not been treated. All possible causes of AF should be looked into and treated as far as possible to reduce arrhythmia recurrence. Also, many drugs as shown in Table 3 are available that can be used to maintain sinus rhythm and prevent recurrence of AF. Even if AF recurs, its frequency or duration may be reduced by long-term use of these agents. However, it should be borne in mind that the efficacy of these drugs is at the most modest and silent asymptomatic AF episodes occur commonly. It is important to realize that even when sinus rhythm has been achieved and maintained, risk of thromboembolism and stroke persists and anti-thrombotic therapy as per the recommendations should be continued indefinitely.
The selection of a particular drug is largely based on safety profile. The drugs like flecainide and propafenone should be avoided in patients with coronary disease, significant left ventricular hypertrophy, or systolic dysfunction and heart failure. Other factors like risk for bradyarrhythmias, renal and liver function, and risk of QT prolongation guide the choice of the drug. Amiodarone is the only safe drug in patients with heart failure or left ventricular systolic dysfunction but periodic monitoring for non-cardiac toxicity is essential during long-term use.
Table 3. Drugs for maintenance of sinus rhythm in atrial fibrillation
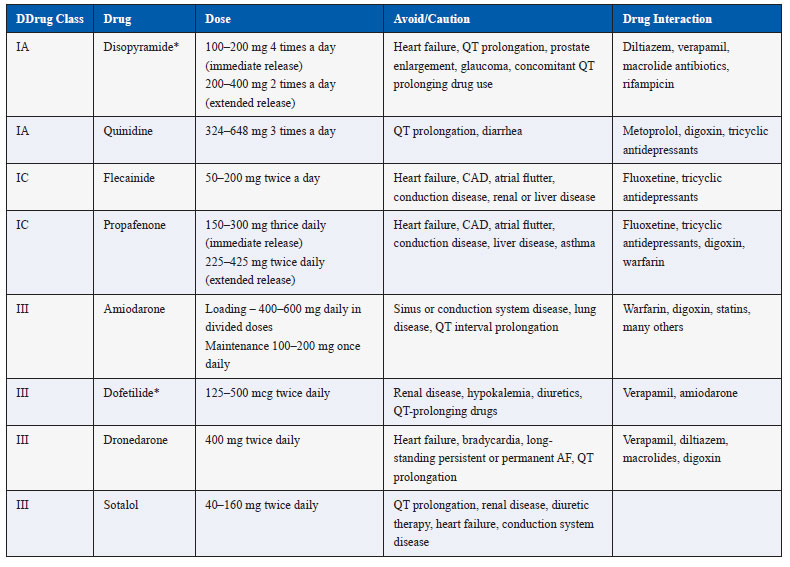
*Not available in India
Catheter Ablation for Atrial Fibrillation
Radiofrequency catheter ablation for AF management has evolved over the last 15 years or so with improvement in efficacy and safety. It is based on electrical isolation of the pulmonary veins that harbor the triggers of AF initiation, with or without modification of atrial substrate (22). Newer approaches like using cryoballoon ablation and multipoint radiofrequency ablation are being evaluated to make the procedure of pulmonary vein isolation simpler and more effective (23). The procedure is complex, has a learning curve, often requires advanced equipment, and can be associated with serious complications like stroke or TIA, left atrial-esophageal fistula, vascular access complications, pericardial effusion, and tamponade and even death (24–26). The efficacy of catheter ablation still has more scope for improvement since it is at the most 70–80% for paroxysmal AF (27). The rate of freedom from AF declines as the follow-up duration becomes longer and often more than one procedure is needed. The efficacy is better for paroxysmal AF compared to persistent AF and also reduced as the duration of AF increases. The AF ablation procedure should be definitely considered in young patients with marked symptoms who do not have significant heart disease and have either failed AAD therapy or are not desirous of long-term drug therapy (27).
Pacemaker Therapy for AF
Often patients with AF also have symptomatic bradycardia either spontaneously or as a result of drugs required for management of AF. These patients need implantation of pacemakers that also enables better drug management of AF as risk of bradycardia is taken care of. The pacemakers should be atrial-based (usually dual-chamber) to prevent the risk of increase in AF occurrence as a result of unnecessary ventricular pacing (28). Many pacemakers have advanced algorithms to prevent AF and reduce its frequency that can be turned on in patients with AF. However, the efficacy of these algorithms in prevention of AF is inconsistent, and hence pacing is not indicated for sole purpose of prevention of AF in patients who do not have other indications of pacing.
Atrial defibrillators that automatically cardiovert AF have gone into disrepute because of pain and discomfort associated with shocks and unproven clinical value
Choosing Rate-Control or Rhythm-Control Strategy
An initial rate-control strategy is reasonable in most patients with AF. However, some patients with AF continue to remain markedly symptomatic and have poor quality of life despite rate control (29,30). Moreover, adequate rate control may be difficult to achieve with existing drugs in many patients. In many other clinical scenarios, rhythm-control strategy may be the preferred approach despite hard clinical evidence for the same. These include young age, first episode of AF, AF secondary to acute treatable illness, tachycardiomyopathy, and when patient prefers rhythm control. The atrial substrate remodels when AF persists for longer term and AF progresses from paroxysmal to persistent in many patients. The structural and electrical alterations in the atrial myocardium become irreversible with time making rhythm control more difficult as the duration of AF increases (31,32). Hence, early intervention with a rhythm-control strategy to prevent the progression of AF may be beneficial in some patients (33).
On the other hand, rate-control strategy may be the preferred approach in patients who have minimal symptoms, are elderly, or have low chances of maintaining sinus rhythm like markedly dilated atria or very long-standing AF.
References
- Brignole M, Menozzi C, Gianfranchi L, Musso G, Mureddu R, Bottoni N, Lolli G. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological treatment in patients with heart failure and chronic atrial fibrillation: a randomized, controlled study. Circulation. 1998;98:953–60.
- Kay GN, Ellenbogen KA, Giudici M, Redfield MM, Jenkins LS, Mianulli M, Wilkoff B. The Ablate and Pace Trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation. APT Investigators. J Interv Card Electrophysiol. 1998;2:121–35.
- Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, Shinn T, St. John Sutton M, for the Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–93.
- Olshansky B, Rosenfeld LE, Warner AL, Solomon AJ, O’Neill G, Sharma A, Platia E, Feld GK, Akiyama T, Brodsky MA, Greene HL; AFFIRM Investigators. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 2004;43:1201–8.
- Platia EV, Michelson EL, Porterfield JK, Das G. Esmolol versus verapamil in the acute treatment of atrial fibrillation or atrial flutter. Am J Cardiol. 1989;63:925–9.
- Farshi R, Kistner D, Sarma JS, Longmate JA, Singh BN. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: a crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999;33:304–10.
- Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–40.
- Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace. 2011;13:308–28.
- Goette A, Schon N, Kirchhof P, Breithardt G, Fetsch T, Häusler KG, Klein HU, Steinbeck G, Wegscheider K, Meinertz T. Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circ Arrhythm Electrophysiol. 2012;5:43–51.
- Berger M, Schweitzer P. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol. 1998;82:1545–7, A8.
- Airaksinen KE, Gronberg T, Nuotio I, Nikkinen M, Ylitalo A, Biancari F, Hartikainen JE. Thromboembolic complications after cardioversion of acute atrial fibrillation – The FinCV Study. JACC. 2013;62:1187–92.
- Jaber WA, Prior DL, Thamilarasan M, Grimm RA, Thomas JD, Klein AL, Asher CR. Efficacy of anticoagulation in resolving left atrial and left atrial appendage thrombi: a transesophageal echocardiographic study. Am Heart J. 2000;140:150–6.
- You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, Hylek EM, Schulman S, Hughes M, Spencer FA, Manning WJ, Halperin JL, Lip GYH. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531S–e575S.
- Klein AL, Grimm RA, Murray RD, Apperson-Hansen C, Asinger RW, Black IW, Davidoff R, Erbel R, Halperin JL, Orsinelli DA, Porter TR, Stoddard MF; Assessment of Cardioversion Using Transesophageal Echocardiography Investigators. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411–20.
- Weigner MJ, Thomas LR, Patel U, Schwartz JG, Burger AJ, Douglas PS, Silverman DI, Manning WJ. Early cardioversion of atrial fibrillation facilitated by transesophageal echocardiography: short-term safety and impact on maintenance of sinus rhythm at 1 year. Am J Med. 2001;110:694–702.
- Weigner MJ, Caulfield TA, Danias PG, Silverman DI, Manning WJ. Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann Intern Med. 1997;126:615–20.
- January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014 Apr 10. [Epub ahead of print]
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation – developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;(10):1385–413
- Oral H, Souza JJ, Michaud GF, Knight BP, Goyal R, Strickberger SA, Morady F. Facilitating transthoracic cardioversion of atrial fibrillation with ibutilide pretreatment. N Engl J Med. 1999;340:1849–54.
- Alboni P, Botto GL, Baldi N, Luzi M, Russo V, Gianfranchi L, Marchi P, Calzolari M, Solano A, Baroffio R, Gaggioli G. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the pocket” approach. N Engl J Med. 2004;351:2384–91.
- Khan IA. Single oral loading dose of propafenone for pharmacological cardioversion of recent-onset atrial fibrillation. J Am Coll Cardiol. 2001;37:542–7.
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen S-A, Crijns HJG, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim Y-H, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao H-M, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–96.
- Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, Reddy V, Nelson L, Holcomb RG, Lehmann JW, Ruskin JN; STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) Pivotal Trial. J Am Coll Cardiol. 2013;61:1713–23.
- Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated Bhargava K Atrial Fibrillation Part II: Rate and Rhythm-Control Strategies of Management
- worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8.
- Arbelo E, Brugada J, Hindricks G, Maggioni A, Tavazzi L, Vardas P, Anselme F, Inama G, Jais P, Kalarus Z, Kautzner J, Lewalter T, Mairesse G, Perez-Villacastin J, Riahi S, Taborsky M, Theodorakis G, Trines S; Atrial Fibrillation Ablation Pilot Study Investigators. ESC-EURObservational Research Programme: the Atrial Fibrillation Ablation Pilot Study, conducted by the European Heart Rhythm Association. Europace. 2012;14:1094–103.
- Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2012;59:143–9.
- Leong-Sit P, Zado E, Callans DJ, Garcia F, Lin D, Dixit S, Bala R, Riley MP, Hutchinson MD, Cooper J, Gerstenfeld EP, Marchlinski FE. Efficacy and risk of atrial fibrillation ablation before 45 years of age. Circ Arrhythm Electrophysiol. 2010;3:452–7.
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA 3rd, Ferguson TB Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD; American College of Cardiology Foundation;American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6–e75.
- Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, Fletcher RD, Sharma SC, Atwood JE, Jacobson AK, Lewis HD Jr, Raisch DW, Ezekowitz MD; Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T) Investigators. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–72.
- Hagens VE, Ranchor AV, Van SE, Bosker HA, Kamp O, Tijssen JG, Kingma JH, Crijns HJ, Van Gelder IC; RACE Study Group. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol. 2004;43:241–7.
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68.
- de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA, Crijns HJ. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–31.
- Cosio FG, Aliot E, Botto GL, Heidbüchel H, Geller CJ, Kirchhof P, De Haro JC, Frank R, Villacastin JP, Vijgen J, Crijns H. Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace. 2008;10:21–7.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528