The Journal of Clinical and Preventive Cardiology has moved to a new website. You are currently visiting the old
website of the journal. To access the latest content, please visit www.jcpconline.org.
Mini-Review
A Case of Mixed Dyslipidemia
Volume 4, Oct 2015
Peeyush Jain, MD (Medicine), DM (Cardiology), Ashok Seth, FRCP (London), FRCP (Edinburgh), FRCP (Ireland), FACC, FESC, FSCAI (USA), FIMSA, FCSI, D.Sc. (Honoris Causa), D.Litt (Honoris Causa) New Delhi, India
2015;4(4):98-102
Clinical ProfileA 58-year-old male with stable angina pectoris CCS class II presents with hypertension, type 2 diabetes mellitus, dyslipidemia, and normal kidney function (BP 154/88 mmHg, HbA1C 7.8%, TC 182 mg/dl, LDL-C 108 mg/dl, HDL-C 28 mg/dl, triglycerides 324 mg/dl, and serum creatinine 0.9 mg/dl). He denies family history of premature cardiovascular disease. He is physically active, vegetarian, non-smoker, self-reported moderate drinker and overweight (BMI 27.0). He has been receiving aspirin 75 mg a day, telmisartan 40 mg/d, metoprolol succinate 100 mg/d, atorvastatin 20 mg/d, metformin 1000 mg twice a day, and sitagliptin 100 mg/d. He has no known drug allergy and did not have any surgical operation in past. Physical examination is unremarkable except firm, palpable liver 2½ fingers below costal margin.
Study Questions
Do we need to improve his lipid profile? If yes, what improvement is needed, how should we do it and what clinical outcomes are expected?
Do We Need to Improve his Lipid Profile?
This is a case of mixed dyslipidemia, partially controlled on a moderate dose of atorvastatin. Further improvement in lipid profile is desirable because:
- The patient has clinical atherosclerotic vascular disease.
- He has multiple risk factors (Age >55, male, hypertension, diabetes, and overweight).
- His glycemic control is suboptimal.
- Primary therapeutic target (LDL-C level) has not been achieved.
- Low HDL-C level is an independent risk factor for atherosclerotic disease.
- Hypertriglyceridemia may also be an independent risk factor.
This patient needs not only an improvement in lipid profile but also better control of blood pressure, blood glucose, and weight. Achievement of euglycemia and weight reduction per se may improve his lipid status at least partially, whereas a more judicious choice of anti-hypertensives and anti-anginals may also make a difference. To begin with targets should be set and discussed with the patient (Table 1). Here a word about plasma triglycerides and cardiovascular and all-cause mortality is not out of place. Elevated serum triglyceride levels are dose-dependently associated with higher risks of cardiovascular and all-cause mortality, as compared to the reference triglyceride levels (90–149 mg/dl) (1). Though ATP III guidelines suggest a normal serum triglyceride level to be <150 mg/dl, an American Heart Association (AHA) 2011 scientific statement suggested an optimal triglycerides levels to be below 100 mg/dl (2).
.jpg)
How Should We Achieve these Lipid Targets?
This will need a two stepped approach. One is retrieval of additional clinical information and second is a critical analysis of current treatment and consideration of efficacy, safety, and outcomes of additional treatments, if any.
What Additional Clinical Information May be Needed?
The stated history does not shed any light on thyroid status, total dietary energy intake, specific food habits (e.g. sugar and refined carbohydrate intake and omega 3 fat intake), undesirable drinking patterns (e.g. binge drinking), liver function, cause of hepatomegaly and serum creatine phosphokinase(CPK) level. The importance of these is obvious but two issues deserve special mention. One is drinking patterns. This should be adjudged objectively in all patients who confess to drinking alcohol. Excessive and binge drinking is seldom volunteered unless inquired about. This may be facilitated by CAGE Questionnaire for Alcohol (Table 2) (3). The second issue is the cause of hepatomegaly. Asymptomatic fatty liver is most commonly due to fatty liver that may be an important indicator of metabolic syndrome in such a patient.

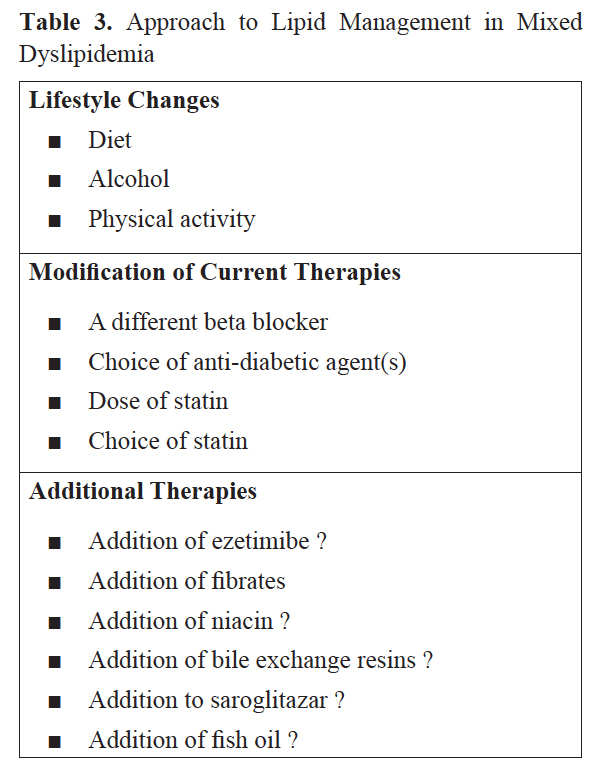


Will Change to a Different Beta Blocker (ββ) Help?
In other words, does any other ββ have an edge over metoprolol in context of dyslipidemia? Such a ββ should be approved for stable angina and substitution should appreciably improve the lipid status. US FDA has approved atenolol, metoprolol, propranolol, and nadolol for management of stable angina pectoris. Bisoprolol is approved in EU and carvedilol is approved in the UK. Nebivolol is not approved for angina in any country but off label use is common (Table 4).
.jpg)
.jpg)
Broadly, cardio-selective and non-selective ββs without intrinsic sympathomimetic activity (ISA) equally increase mean plasma triglycerides by 10-20% and decrease the HDL-C by 7-10% (4). Carvedilol may be an exception. It is reported to be effective in stable angina (5). It appears to be as effective as metoprolol in reducing the frequency of angina and improving exercise duration (6). Twelve published studies and case reports have examined carvedilol and lipid profile (7). Six studies compared carvedilol with cardio-selective ββs. Three compared carvedilol with other antihypertensive medications. Carvedilol alone was evaluated in 3 small studies. In 4 of the 12 studies, carvedilol independently improved lipid profile significantly with a neutral effect in 3 studies. In 3 of the 4 studies, where carvedilol was compared with other medications, other drugs worsened lipid profile significantly compared with carvedilol. Cardio-selective ββs worsen the lipid profile compared with carvedilol.
There are some signals that substituting carvedilol for metoprolol may improve lipid profile. Gemini Study randomized 1235 patients with type 2 diabetes and hypertension to carvedilol, or metoprolol for 5 months (8). In metoprolol group, triglycerides and non-HDL-C increased and LDL-C and HDL-C decreased. In the carvedilol group, LDL-C, HDL-C, and triglycerides decreased. Between 2 groups there was greater decrease in triglycerides with carvedilol (−9.8%). Though lipids were secondary end points in this study, there were encouraging signs for carvedilol instead of metoprolol in this study.
Are there Important Differences among Anti-diabetic Drugs in their Effects on Lipid Profile?
A systemic review of 140 randomized controlled trials and 26 observational studies by US Agency for Healthcare Research and Quality concluded that metformin reduces LDL-C, sulphonylureas and DPP-4 inhibitors have little effect, while glitazones increase LDL-C levels (rosiglitazone > pioglitazone). Pioglitazone increases HDL-C more than rosiglitazone, metformin, or sulphonylureas. Reduction in triglycerides is more marked with pioglitazone than with metformin, sulphonylureas, and rosiglitazone (9). These differences are small however, and better control of blood glucose is probably more important than choice of anti-diabetic drugs when improvement in lipid profile is the goal.
Will Increasing the Dose of Atorvastatin Improve Lipid Profile?
This patient is receiving 20 mg atorvastatin a day and his present LDL-C is 108 mg/dl. Increasing the dose to 40 or 80 mg a day is likely to reduce LDL-C to <100 mg/dl or 94 mg/dl respectively by applying the ‘rule of 6.’ It is not likely to fall below 70 mg/dl, if that is the goal. The ‘rule of 6’ refers to 6% additional reduction in LDL-C for every doubling of statin dose. Of course, there are individual variations and some patients may exhibit a much larger response and achieve their LDL-C target. Nevertheless, it is highly unlikely that low HDL-C and high triglycerides will also be reversed by increasing the dose of atorvastatin.
Does Switching over to Rosuvastatin Increase the Likelihood of Achieving LDL-C Target As Well As Increase HDL-C and Decrease Triglycerides?
Rosuvastatin is an attractive choice as it is more potent than atorvastatin (10). Top dose of 40 mg a day may decrease LDL-C by as much as 50-55% over the baseline (it should be noted that the present LDL-C of 108 mg/dl is not the baseline LDL-C of this patient) and may also increase HDL-C by 7.7% - 9.6%, which is better than high dose atorvastatin. The latter may paradoxically reduce HDL-C below pre-treatment levels, though this often occurs in the setting of marked LDL-C lowering. Effects of rosuvastatin on triglycerides are less substantial (11).
How About Adding Ezetimibe to Atorvastatin?
Ezetimibe monotherapy is not very effective in reducing LDL-C but marked reduction in LDL-C may be observed in combination with statin (12). Some studies showed that a combination of ezetimibe 10 mg/d with atorvastatin 10 mg/d may decrease LDL-C as much as atorvastatin 80 mg a day alone(“10 + 10 = 80”). The recent finding of reduction in the primary end point of cardiovascular death, MI, documented unstable angina requiring rehospitalization, coronary revascularization (≥30 days), or stroke by addition of ezetimibe to simvastatin in IMPROVE IT Study makes a combination of ezetimibe and atorvastatin attractive in this patient (13). Nevertheless, ezetimibe has little effects on HDL-C and triglycerides, if any.
What is the Role of Fibrates in Such a Patient?
A meta-analysis concluded that fibrates reduce major coronary events but do not reduce cardiovascular or total mortality (14). The effect of adding fenofibrate to simvastatin in type 2 diabetes was examined in the open-label ACCORD Lipid Study (n=5518). No difference in outcome was observed in the primary end point of non-fatal MI, nonfatal stroke, or CV death after a mean follow-up of 4.7 years despite significant improvements in HDL-C and triglyceride levels in fenofibrate group (15). A subgroup analysis of patients with high triglycerides (>204 mg/dl) and low HDL-C (<34 mg/dl) had an improved outcome with fenofibrate. This latter hypothesis generating finding is utilized by some physicians to add fenofibrate to statin therapy in mixed diabetic dyslipidemia but a randomized controlled trial in this set of patients is lacking.
How about Adding Nicotinic Acid in this Patient?
After publication of AIM-High and HPS 2 Thrive, both with negative outcomes, and unfavorable side effect profile, there is little enthusiasm for niacin in dyslipidemia management. Nevertheless, this drug may be of some utility in statin-intolerant patients.
Saroglitazar is a Comparatively New Drug, being Promoted for Diabetic Dyslipidemia
Saroglitazar is a dual PPAR alpha/gamma agonist. In a phase III clinical trial (Press VI), saroglitazar reduced triglycerides by 45% and non-HDL by 32%. It also improved insulin sensitivity and glycemic parameters. Statin plus saroglitazar may be effective in management of mixed diabetes dyslipidemia but its large-scale use is limited by lack of outcome data.
What Can be Achieved by Adding Fish Oil to Statin Therapy in this Patient?
Eicosapantenoic acid (EPA) and docosahexanoic acid (DHA) in fish oils lower serum triglycerides by decreasing very low-density lipoporotein (VLDL) synthesis. EPA and DHA may each have different or complementary effects; combined intake appears to
be prudent. Response to fish oils depends on the dose. A daily intake of up 10 g of EPA or DHA is required for maximal reduction of plasma triglycerides. Fish oils may raise LDL levels. Some observational studies suggest benefits of fish intake on clinical outcomes, but intervention trials have not established these benefits. Considering these facts, fish oils should be reserved for patients with severe, refractory hypertriglyceridemia (16). It does not seem an ideal choice for this patient.
Conclusions: How Will I Further Manage Dyslipidemia in this Patient?
After considering all issues, I will first discuss the importance of a low calorie diet, limiting alcohol intake, weight reduction, and better glycemic control with the patient. I may like to switch over to carvedilol instead of metoprolol and add pioglitazone or background insulin for better diabetes control. In all probability, I will switch over to rosuvastatin and up-titrate its dose to achieve LDL-C target. I will expect low HDL-C and high triglycerides to improve over next several months with these measures, only then will I consider addition of fenofibrate.
References
References
- Liu J, Zeng FF, Liu ZM, Zhang CX, Ling WH, Chen YM. Effects of blood triglycerides on cardiovascular and all-cause mortality: a systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis. 2013;12:159.
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292-333.
- Ewing JA. Detecting alcoholism: The CAGE Questionnaire. JAMA. 1984;252:1905-7.
- Lehtonen A. Effect of beta blockers on blood lipid profile. Am Heart J. 1985;109:1192-6.
- Chatterjee K, Topol EJ. Cardiac Drugs. Drugs for Stable Angina. Jaypee, New Delhi. 2013:438.
- van der Does R, Hauf-Zachariou U, Pfarr E, Holtbrügge W, König S, Griffiths M, Lahiri A. Comparison of safety and efficacy of carvedilol and metoprolol in stable angina pectoris. Am J Cardiol. 1999;83:643-9.
- Sharp RP, Sirajuddin R, Sharief IM. Impact of carvedilol on the serum lipid profile. Ann Pharmacother. 2008;42:564-71.
- Bell DS, Bakris GL, McGill JB. Comparison of carvedilol and metoprolol on serum lipid concentration in diabetic hypertensive patients. Diabetes, Obesity, and Metabolism. 2009;11:234-8.
- Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Block L, Nicholson WK, Hutfless S, Bass EB, Bolen S. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-13.
- Olsson AG, Istad H, Luurila O, Ose L, Stender S, Tuomilehto J, Wiklund O, Southworth H, Pears J, Wilpshaar JW; Rosuvastatin Investigators Group. Rosuvastatin Study Group. Effects of rosuvastatin and atorvastatin compared over 52 weeks of treatment in patients with hypercholesterolemia. Am Heart J. 2002;144:1044-051.
- Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, Cain VA, Blasetto JW; STELLAR Study Group. Stellar Study Group. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR Trial). Am J Cardiol. 2003;92:152-60.
- Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP; Ezetimibe Study Group. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107:2409-15.
- Murphy SA, Cannon CP, Blazing MA, Giugliano RP, White JA, Lokhnygina Y, Reist C, Im K, Bohula EA, Isaza D, Lopez-Sendon J, Dellborg M, Kher U, Tershakovec AM, Braunwald E. Reduction in Total Cardiovascular Events With Ezetimibe/Simvastatin Post-Acute Coronary Syndrome: The IMPROVE-IT Trial. J Am Coll Cardiol. 2016;67:353-61.
- Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J, Perkovic V. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis Lancet. 2010;375:1875-84.
- ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, et. al. Effects of combination lipid therapy in type 2 diabetes mellitus. NEJM. 2010;362:1563-74.
- Mozaffarian D. Nutrition and Cardiovascular and Metabolic Diseases in Braunwald’s Text Book of Cardiology. 10th Edn. 2015:997-1008.
- Why Publish with JCPC?
- Instructions to the Authors
- Submit Manuscript
- Advertise with Us
- Journal Scientific Committee
- Editorial Policy
Print: ISSN: 2250-3528


